Category: News
¹⁶¹Tb-PSMA: New Hope for Patients with Metastatic Prostate Cancer
¹⁶¹Tb-PSMA: New Hope for Patients with Metastatic Prostate Cancer
VIOLET Study – First Results
Coinciding with the launch of the therapy at our center, the early results of the VIOLET study, the first-in-human trial of ¹⁶¹Tb-PSMA, are being published. And they are highly promising! No dose-limiting toxicities were observed across six treatment cycles (up to 7.4 GBq) in 30 patients. Encouraged by these results, a new cohort at 9.5 GBq has been initiated.
¹⁷⁷Lu-PSMA radioligand therapy has proven highly effective for prostate cancer, but some patients eventually experience disease progression. A likely reason is the presence of energy-sheltered micrometastases and single tumor cells, which absorb very little radiation due to their small size and limited mass. The beta particles emitted by ¹⁷⁷Lu have a mean pathlength of ~0.7 mm (range: 0.04–1.8 mm), which far exceeds the size of single tumor cells measuring only a few micrometres (7–13 μm). As a result, most radiation overshoots these tiny cancer cells, failing to deposit lethal energy and allowing disease progression.
In contrast, ¹⁶¹Tb emits abundant Auger and conversion electrons, which have much shorter pathlengths (nanometers to micrometers). This enables precise, high-energy deposition directly into single tumor cells and micrometastases, making it potentially more effective than ¹⁷⁷Lu at eradicating these resistant cancer cells. Additionally, ¹⁶¹Tb may further reduce radiation-related toxicity by limiting unnecessary exposure to surrounding healthy tissue.
¹⁶¹Tb-PSMA radioligand therapy is already available at our center.
The VIOLET study is named in honor of the late Dr. John Violet, a radiation oncologist at the Peter MacCallum Cancer Centre in Melbourne, Australia, who had a keen interest in Auger electron therapy. The study aims to determine the maximum tolerated dose, safety profile, and antitumor activity of ¹⁶¹Tb-PSMA in patients with mCRPC. The therapy is administered in an outpatient setting, with key objectives including measuring absorbed radiation doses, PSA response, survival outcomes, and patient-reported quality of life.
Interview with Prof. Hartenbach, conducted by Primo Medico on 31 March 2025
Interview with Prof. Hartenbach, conducted by Primo Medico on 31 March 2025 (English audio AI-generated, original in German)
Primo Medico Specialist Talk, the Specialists’ Podcast with Susanne Amrhein — “Medicine for the Ears”
In prostate cancer, precise and early detection of the tumor and its spread is crucial for subsequent treatment. The gold standard in diagnosis here is the PSMA PET/CT. This also makes it possible to perform targeted and effective radioligand therapy. I talk with Professor Markus Hartenbach about the close connection between therapy and diagnostics — the so-called theranostics — as well as the principles and effects of radioligand therapy. He is a specialist in nuclear medicine and managing director of Minute Medical in Vienna.
- [00:40] Why is PSMA-PET/CT so important?
- [02:55] How does PSMA radioligand therapy work?
- [04:19] Which radionuclides are used?
- [07:00] Why do you offer the therapy on an outpatient basis?
- [09:05] How does your treatment differ from other therapies?
- [10:41] How tolerable is radioligand therapy?
- [12:15] How do you verify treatment success?
- [13:45] Can the therapy be repeated?
PrimoMedico (PM): Professor Hartenbach, why does PSMA-PET/CT play such an important role in the subsequent treatment of prostate cancer?
Prof. Markus Hartenbach (MH): Yes, dear Ms. Amrhein, first of all, thank you very much for organizing this podcast, and warm greetings from Minute Medical here in Vienna.
The PSMA-PET/CT is essentially the gateway to what you’ve already mentioned — theranostics — and in the case of prostate cancer, it’s quite a specific approach. What that means is that we’re looking for a very particular target structure in the body. It’s less about classic imaging and more about visualizing molecular surface markers.
PSMA, or Prostate-Specific Membrane Antigen, is actually a somewhat misleading name, because in reality it’s an enzyme called folate hydrolase. This enzyme can now be specifically visualized, and the PET/CT scanner is the tool that makes this molecular mechanism visible. In this process, radioactive tracers like Gallium-68 or Fluorine-18 are used, which allow me to scan the entire body from head to toe and display this molecule in detail.
In prostate cancer cells, PSMA is overexpressed — meaning there’s a particularly high density of this enzyme on the cell surface. That density also serves as a marker or indicator of how aggressive the tumor is. We know that tumors which overexpress PSMA very strongly — even just in the prostate itself, without any metastases — already have significant prognostic implications for how the disease will develop.
And now, if there’s a sufficiently high overexpression of PSMA, I know I can target it therapeutically. The more PSMA is expressed — and many studies have shown this — the higher the therapeutic dose that can be delivered directly to the cancer cells, because PSMA is internalized by the cells. That allows me to irradiate the cancer cells from the inside, and depending on the radionuclide used, also affect neighboring cells.
This is why PSMA-PET/CT is so crucial — first as the gateway to treatment, and second to assess whether the patient actually has metastases somewhere in the body that are also overexpressing PSMA and don’t belong there. And of course, I can also use this imaging later on, after treatment — whether it’s radioligand therapy, antihormonal therapy, chemotherapy, or radiation — as a follow-up tool to see whether the cancer cells were effectively treated.
PM: What happens during PSMA radioligand therapy?
MH: The PSMA-ligand therapy now takes advantage of the fact that — as I’ve already proven with the PET scan, or in some cases unfortunately not proven, since there can also be PSMA-negative or low PSMA-expressing metastases — that this target structure is, at least assumedly, present in the body.
Now what I do is swap out the radionuclide used in the PET scan — Gallium-68, Fluorine-18, or sometimes Copper — and replace it with a therapeutic radionuclide. This means using a type of radiation emitter that releases what we call corpuscular radiation, or particle radiation. In most cases, when I use Lutetium-177, it’s what we call a beta emitter, meaning it emits electrons. But I could also use other radionuclides — we might get to those in a moment.
The radiopharmaceutical, as we call it, then binds to and is internalized by the cancer cell — in this case, via the PSMA. But this time, the goal is not to visualize it in an image, but to unleash its therapeutic effect.
And here’s what happens — what radiation, whether external or internal, essentially does to a cell: ideally, it begins to destroy the DNA of the cancer cell. The goal is to damage it so extensively that the cell can no longer divide, allowing the body to gradually break down and eliminate the tumor.
PM: You use different radionuclides — when do you choose which one?
MH: Yes — in fact, we offer several different radionuclides. On one hand, there’s Lutetium, a beta emitter that’s already widely established — it’s been used for over a decade in prostate cancer and even longer in other types of tumors because it has excellent properties: the beta radiation itself, its half-life, and its tissue penetration range, which is about one to two millimetres.
But we’ve also found — and this is currently a rapidly growing field of research — that other radionuclides can be extremely effective, sometimes even more effective against cancer cells while being gentler on the surrounding healthy tissue.
One important example here is Actinium, an alpha emitter that’s also been in use for a long time, at least in university settings, and is now increasingly being tested in clinical trials. It’s particularly well-suited for patients with extensive bone marrow involvement. Actinium emits alpha radiation, which has a very short range — about one to two cell diameters in tissue — meaning there’s virtually no collateral damage in areas where it doesn’t accumulate.
The only issue we’ve observed with Actinium-PSMA is that if treatments are repeated often, the salivary glands — which unfortunately also express this enzyme — can suffer damage.
In the bone marrow itself, even in patients with heavy marrow involvement, we see very little collateral damage, possibly even less than with Lutetium, because the radiation range is so short. That’s why we primarily use Actinium in cases with extensive bone marrow metastases.
And just recently, we’ve started offering Terbium-161 as well. This one’s a little more complex. What kind of radiation does Terbium emit? It’s somewhat comparable to Lutetium, with a beta component, though not as strong. But it also emits what are called Auger electrons and conversion electrons — I won’t go too deeply into the details here because it gets quite technical — but these have an ultra-short range.
This means the complex, once internalized into the cell, needs to get very close to the cell nucleus. Once it’s there, these electrons can release extremely high energy, potentially offering an even better chance of destroying the cell nucleus than Actinium’s alpha radiation. And because we don’t need to inject as much radioactivity as we would with Lutetium, there’s likely to be even less collateral damage.
However, we’re still in the early stages here — the first prospective clinical studies are currently underway. In practice, we tend to use Terbium when patients have become refractory to Lutetium and even to Actinium, and when we want to try one more therapeutic option, an individualized treatment attempt, with Terbium — though the available data is still quite limited at this point.
PM: Many clinics offer this therapy as an inpatient procedure, while you treat patients on an outpatient basis. Why?
MH: Cancer has a lot to do with the psyche, and especially at different stages of the disease — and particularly in earlier stages, where radioligand therapy is increasingly being used for prostate cancer — patients don’t want to be reminded of what things might look like in the final stages. They also don’t want to feel like they’re being locked away or treated as seriously ill when, in fact, they don’t feel that way.
Most patients walk into the clinic saying they don’t feel a thing from their prostate cancer, even if the PSMA-PET scan might suggest otherwise by lighting up with black spots all over. That’s actually what inspired us — coming from a clinical background, where we’ve done this therapy for years at the AKH in Vienna (Vienna General Hospital) — to rethink the situation. Until now, the only reason for keeping patients in the hospital was radiation protection, which dictated, “Well, just to be safe, let’s keep them for at least 48 hours,” as is still commonly done in Germany.
But we said: okay, let’s sit down with the authorities, carry out extensive measurements, and show that these patients aren’t actually a radiation threat to their surroundings. That’s what eventually got us the official approval. And in Austria, the trend — especially after our recent annual conference — is clearly moving in this direction. People are saying: of course, outpatient therapy is entirely safe from a radiation standpoint.
Of course, patients are given clear instructions, particularly if they’re going to be in contact with pregnant women or small children — in those cases, they’re advised to keep a bit of distance for about a week. That might mean not visiting the grandchildren or holding off on seeing a pregnant daughter for a few days. These are safety precautions that are easy to follow.
The treatment itself is short — essentially just a brief infusion. And while patients do have to drink plenty of fluids afterwards to help flush the substance out through the kidneys, that’s something any adult can easily manage at home. So there’s really little to no reason to keep relatively healthy patients in a hospital setting for this. It’s a different story, of course, with seriously ill patients — there the situation is handled differently.
PM: How does your therapy differ from other cancer treatments?
MH: Well, sticking to prostate cancer, there are very clear guidelines that have long placed hormone therapy at the start of treatment for metastatic disease. Hormone therapy, in its various forms — whether blocking hormone production or acting as an androgen receptor blocker — absolutely has its place. In practice, patients often see quick results with hormone therapy, but unfortunately, they also start experiencing side effects fairly quickly.
Chemotherapy, of course, is always a topic when it comes to cancer, and in prostate cancer — particularly with drugs like Docetaxel and Cabazitaxel — it has shown rather modest results. The side effects tend to be quite significant, and because prostate cancer typically isn’t a very fast-growing tumor, chemotherapy somewhat lacks a clear target.
Our target, ultimately, is radiation. And while we talk about it as if we were administering a drug — as it’s treated this way by regulatory agencies like the EMA and FDA — in the end, what we’re actually doing is a form of radiotherapy. The pharmaceutical component, the ligand that attaches to the cancer cell, could be administered in concentrations ten thousand times higher and still wouldn’t trigger any reaction in the body. That’s something we confirm through tests beforehand. The only therapeutic effect comes from the radiation. And that radiation can be delivered in different qualities, depending on the type of radionuclide we use. Essentially, it’s like external radiotherapy — but much more precise, because it takes place directly at the cell surface and throughout the entire body. That’s what fundamentally sets our therapy apart from external beam radiation.
PM: How well is radioligand therapy tolerated?
MH: We can actually build on what we just discussed. Because our treatment is extremely precise at the level of the cell surface, we see relatively little collateral damage. And here again comes the theranostic principle into play: first, I look at the PET/CT scan. In the PET/CT, I need to see, first and foremost, that the metastases ideally express PSMA more strongly than the other organs that also have PSMA.
And because — as I mentioned before — it’s somewhat misleadingly named, since PSMA is actually Folate Hydrolase, an enzyme, we unfortunately also find it in the salivary glands. I’ve already pointed that out. The kidneys also receive a certain amount of radiation. The other organs are typically less affected. The bone marrow gets a small dose too, but this is usually reversible. Prospective studies haven’t shown any significant, higher-grade side effects to a meaningful extent.
That said, if we perform many, many therapy cycles — and we have already done quite a few repeat treatments — we can start to see the salivary gland function slowly decrease over time. That’s understandable, because they are also exposed to some radiation.
Kidney function is generally only at risk when combined with other kidney-stressing medications, or if the kidneys already have pre-existing damage — whether from surgery, external radiation, other therapies, chemotherapy, and so forth. So it’s important to consistently monitor kidney function. Proper hydration before, during, and after the therapy is absolutely essential. And with good hydration management, even with multiple treatment cycles, we haven’t seen kidney damage occur.
PM: How do you check whether the treatment was successful?
MH: And here again, our wonderful PSMA-PET/CT comes into play. Within the theranostic concept, I can use the same target — as we call it — to check: is it gone? Ideally, of course, it’s no longer detectable. But we have to remember, we’re dealing with cancer, and cancer is something that can always strike back. There are cells in certain phases of the cell cycle where they’re not susceptible to damage — a bit like fungal spores hiding somewhere, waiting for a weakened immune system to reactivate them. That’s how recurrences can happen.
Naturally, what we’re doing here isn’t the holy grail that cures everything — recurrences can still appear later on. And if we detect something with the PSMA-PET but can’t resolve it properly, that sometimes has to do with the scanner’s resolution. We can’t visualize individual cells — that’s beyond the capability of current imaging — but we can use it as a surrogate marker. If the PSMA-PET no longer shows any activity, and ideally the PSA level is also undetectable — assuming the patient no longer has a prostate, because otherwise normal prostate tissue would still produce some PSA — then we can talk about a complete remission.
We can actually quantify the regression of metastases. We always compare the pre-therapeutic PSMA-PET, measuring both the volume of the metastases and their PSMA expression, which can be measured in the scan. Then we compare that to the post-therapeutic PET. We’ve already published studies showing that this measurement can reliably serve as a surrogate for the patient’s disease progression.
PM: Can radioligand therapy be repeated if the cancer returns?
MH: The answer is quite simple — yes in most cases. I mentioned it briefly earlier: we’ve conducted therapies up to 18 times in some patients. We follow a somewhat stricter protocol here than what’s currently used with the approved drug Pluvicto. We schedule the therapy every four weeks, because back in the days when no approved product was available, we noticed at the university clinic that using this stricter schedule resulted in a somewhat higher response rate compared to other clinics.
That’s why we stick to this stricter protocol. Back then, we also used a slightly higher dose. But after every three cycles, we performed a PET scan, and if we saw the patient was in remission, we’d allow the body to take over for a while and sustain that remission — which very often led to excellent outcomes.
If, after a longer interval, there was still something detectable, or if a new metastasis appeared, we would start treating again. So essentially, we always worked in blocks of three sessions. We’ve kept this approach here in our private practice as well.
In practical terms, if the patient isn’t experiencing major side effects — particularly concerning the bone marrow or kidneys — it’s absolutely possible to carry out up to 20 treatments. In fact, a recent paper in The Journal of Nuclear Medicine reported on a case with 22 therapies in one patient. So yes — it’s possible.
PM: Thank you, Professor Hartenbach.
MH: My pleasure.
FDA Approves ¹⁷⁷Lu-PSMA Before Chemotherapy
FDA Approves ¹⁷⁷Lu-PSMA for mCRPC Before Chemotherapy
The FDA has expanded the indication for ¹⁷⁷Lu-PSMA-617 (Pluvicto®), now approving its use before chemotherapy in patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC).
Eligible patients must have:
- PSMA PET-positive disease
- disease progression on androgen deprivation therapy (ADT) and one androgen receptor pathway inhibitor (ARPI)
The decision is based on positive results from the phase 3 PSMAfore trial (NCT04689828).
Pluvictory: collaboration with the Croatian Health Insurance Fund (HZZO) on Pluvicto in outpatient setting
Pluvictory: collaboration with the Croatian Health Insurance Fund (HZZO) on Pluvicto in outpatient setting
We are pleased to announce our collaboration with the Croatian Health Insurance Fund (Hrvatski Zavod Za Zdravstvene Osiguranje), which now enables its patients to access Pluvicto® (PSMA radioligand therapy) within the approved indication in an outpatient setting!
This is a great step for patient care—but just a small step for the patient, as outpatient therapy eliminates the need for hospitalization, with each session lasting only about one hour.
With the recent FDA approval of Pluvicto for use before chemotherapy, we hope that EMA and other public health insurers in Europe will soon follow.
💡 Outpatient, patient-friendly, effective!
What if my oncologist doesn’t recommend ¹⁷⁷Lu-PSMA?
What if my oncologist doesn’t recommend ¹⁷⁷Lu-PSMA?
Refuting Oncologist’s Doubts About Radioligand Therapy: A Case for Confidence
As ¹⁷⁷Lu-PSMA therapy gains attention for its role in treating metastatic castration-resistant prostate cancer (mCRPC), some oncologists remain hesitant to fully embrace it. Their concerns, while understandable, are often based on regulatory, safety, and patient selection uncertainties. However, a growing body of evidence and clinical experience suggests that many of these doubts are unfounded, and it’s time to address them head-on.
- Regulatory Approval: Procedural Lag & Geographic Differences
While ¹⁷⁷Lu-PSMA (Pluvicto© by Novartis) was approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as a late-stage treatment for metastatic castration-resistant prostate cancer, one of the most frequent arguments against the widespread adoption of ¹⁷⁷Lu-PSMA therapy is the lack of formal approval at earlier stages. Without regulatory approval, oncologists express concerns over the absence of established inclusion criteria and treatment guidelines. However, this objection overlooks several important factors:
- Precedent from Lutathera: Radioligand therapy (RLT) is not a new concept. ¹⁷⁷Lu-DOTATATE (Lutathera©), a similar radioligand treatment for neuroendocrine tumors, was approved by the EMA in 2017 and by the FDA in 2018 for patients with locally advanced or metastatic disease that is unresectable or progressive. Its success set a clear path for the approval of ¹⁷⁷Lu-PSMA. The PSMA-targeted approach is based on the same theranostic principle, combining diagnosis and therapy, and has benefited from nearly a decade of clinical application in countries like Germany. This track record builds confidence in its therapeutic framework.
- Flexible Access: While the medical regulatory framework in the US is stricter, many countries have developed a more flexible and patient-centred approach, where doctors have greater medical liberty to recommend treatments based on individual patient needs, such as intolerance to chemotherapy or hormonal treatments. This approach has greatly expanded access to ¹⁷⁷Lu-PSMA, even at earlier disease stages. The accumulated experience has been invaluable in refining patient selection criteria and demonstrating safety in real-world clinical settings. Germany, for instance, has led the charge in integrating this therapy into their prostate cancer treatment landscape at various stages with highly promising results.
- Clinical Trials: Rigorous clinical trials, including PSMAfore and UpFrontPSMA, continue to accumulate robust data on the safety and efficacy of ¹⁷⁷Lu-PSMA. These studies are critical to advancing the therapy to earlier stages, and their outcomes support the use of this therapy as a front-line option in patients with high PSMA expression.
- Adverse Effects: Manageable and Comparable to Conventional Therapies
Concerns over adverse effects, particularly nephrological and haematological toxicities such as microangiopathy or thrombocytopenia, are often cited by oncologists as reasons to hesitate in applying ¹⁷⁷Lu-PSMA therapy. However, the data we have thus far indicates that the side effect profile is not only manageable but, in many cases, far less severe than that associated with conventional treatments like chemotherapy and external radiation. Besides, in such a disease as cancer “it is not reasonable to define absolute contra-indications. In general, the chances to improve should outweigh the risks of harming a patient.” [1]
- High Precision: The key advantage of ¹⁷⁷Lu-PSMA, or any radioligand therapy for that matter, is its ability to selectively target cancer cells while sparing healthy tissue, reducing off-target effects. Chemotherapy, on the other hand, has a well-known systemic impact, causing fatigue, nausea, and significant haematologic side effects such as neutropenia and anaemia, which can be far more debilitating. Similarly, external radiation can lead to localized damage to healthy organs surrounding the tumor. In contrast, ¹⁷⁷Lu-PSMA’s targeted approach minimizes these risks and actually improves the quality of life, as demonstrated in multiple trials and compassionate use settings.
The main challenge is the natural presence of PSMA enzymes in other organs like the salivary glands. The name "Prostate" in PSMA (Prostate Specific Membrane Antigen) is to an extent misleading; while this enzyme was indeed first discovered on the surface of normal prostate cells, it is actually present in greater numbers in other organs. The most common side effect after ¹⁷⁷Lu-PSMA therapy is xerostomia or dry mouth. However, studies and experience have shown that this is mostly transient and reversible and is far outweighed by the therapeutic benefits. Moreover, doctors have developed prophylactic precautions to mitigate these expected side-effects by accompanying intravenous saline infusions to maintain sufficient hydration and if needed protective co-medication.
- Unclear Source of Side Effects: Given that most novel therapeutic approaches first involve late-stage, heavily pre-treated patients, it is difficult to definitively attribute some side effects like thrombocytopenia to the therapy itself or to the progression of advanced cancer. In fact, there is growing evidence that administering ¹⁷⁷Lu-PSMA earlier in the disease course — before patients undergo multiple rounds of chemotherapy or hormonal treatment — could reduce the incidence of side effects and further enhance the therapeutic effect, as healthier bone marrow and immune systems are better equipped to tolerate the therapy and even combat the cancer weakened by it.
- Patient Selection Criteria: Gaining Consensus
Another area of ongoing debate is how to best select patients for ¹⁷⁷Lu-PSMA therapy. Some oncologists argue that without universal criteria, treatment protocols will remain inconsistent, leading to variable outcomes. However, the selection process for ¹⁷⁷Lu-PSMA therapy is actually clearer than for many other treatments. The field is, after all, called "theranostics" for a reason.
- PSMA PET Imaging: PSMA enzyme first established itself as a diagnostic target. The integration of ⁶⁸Ga-PSMA PET/CT imaging as part of the pre-treatment assessment protocol was a game-changer. It enables the detection of micrometastases that are not “visible” with traditional diagnostic methods such as ¹⁸F-FDG or scintigraphy, ensuring precise staging of the disease. This imaging modality provides a detailed evaluation of PSMA expression, ensuring that only patients with high uptake are considered for therapy. In fact, “PSMA-PET (or PSMA-SPECT) is a strong and relative unique factor for prediction of an individual patient’s response probability to 177Lu-PSMA-RLT”.[1] As more institutions adopt this tool, patient selection will become more consistent, and variability in treatment outcomes will decrease.
- Experience in Germany and Australia: Countries like Germany and Australia, which have been at the forefront of ¹⁷⁷Lu-PSMA research and application, have accumulated a wealth of experience over the past decade. This experience shows that selecting patients based on PSMA expression and prior treatment history leads to highly effective outcomes. Not for nothing is it called "personalized medicine".
- The Case for Early Intervention
UpFrontPSMA and PSMAfore trials, have shown that the earlier ¹⁷⁷Lu-PSMA is introduced, the better the outcomes, with a more significant reduction in PSA levels and fewer long-term side effects compared to later-stage applications.
Conclusion: Confidence in a Proven Modality
While concerns about regulatory approval, side effects, and patient selection are valid in any new therapy, the evidence surrounding ¹⁷⁷Lu-PSMA therapy strongly supports its safety and efficacy. With over a decade of experience in several countries, ongoing trials, and the successful application of similar therapies like Lutathera, the case for radioligand therapy as a standard-of-care option is compelling. If your oncologist is still hesitant, feel free to put them in touch with us. Cancer requires an interdisciplinary approach.
Bibliography
177Lu-PSMA-617 radioligand therapy of metastatic castration-resistant prostate cancer: Initial 254-patient results from a prospective registry (REALITY Study). Khreish F, Ghazal Z, Marlowe RJ, Rosar F, Sabet A, Maus S, Linxweiler J, Bartholomä M, Ezziddin S. Eur J Nucl Med Mol Imaging. 2022 Feb;49(3):1075-1085. doi: 10.1007/s00259-021-05525-7. Epub 2021 Sep 7. PMID: 34494131; PMCID: PMC8803625.
[177Lu]Lu-PSMA-Radioligand Therapy Efficacy Outcomes in Taxane-Naïve Versus Taxane-Treated Patients with Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Metaanalysis. Swayamjeet Satapathy, Ranjit K. Sahoo, Chandrasekhar Bal, Journal of Nuclear Medicine May 2023, 265414; DOI: 10.2967/jnumed.123.26541
Comparative Analysis of Morphological and Functional Effects of 225Ac- and 177Lu-PSMA Radioligand Therapies (RLTs) on Salivary Glands. Feuerecker, Benedikt & Gafita, Andrei & Langbein, Thomas & Tauber, Robert & Seidl, Christof & Bruchertseifer, Frank & Gschwendt, Jürgen & Weber, Wolfgang & D'Alessandria, Calogero & Morgenstern, Alfred & Eiber, Matthias. (2023). International Journal of Molecular Sciences. 24. 16845. 10.3390/ijms242316845.
Extensive 177Lu-PSMA Radioligand Therapy Can Lead to Radiation Nephropathy with a Renal Thrombotic Microangiopathy–like Picture. Schäfer, Hannah & Mayr, Sarah & Büttner, Maike & Knorr, Karina & Steinhelfer, Lisa & Böger, Carsten & Gschwend, Jürgen & Heemann, Uwe & Eiber, Matthias & Schmaderer, Christoph & Tauber, Robert. (2022). European Urology. 83. 10.1016/j.eururo.2022.05.025.
Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Kratochwil, Clemens & Fendler, Wolfgang & Eiber, Matthias & Hofman, Michael & Emmett, Louise & Calais, Jeremie & Osborne, Joseph & Iravani, Amir & Koo, Phillip & Lindenberg, Liza & Baum, Richard P. & Bozkurt, Murat & Delgado Bolton, Roberto C. & Ezziddin, Samer & Forrer, Flavio & Hicks, Rodney & Hope, Thomas & Kabasakal, Levent & Konijnenberg, Mark & Hermann, Ken. (2023). European Journal of Nuclear Medicine and Molecular Imaging. 50. 10.1007/s00259-023-06255-8.
Long-Term Nephrotoxicity of 177Lu-PSMA Radioligand Therapy. Steinhelfer, Lisa & Lunger, Lukas & Cala, Lisena & Pfob, Christian & Lapa, Constantin & Hartrampf, Philipp & Buck, Andreas & Schäfer, Hannah & Schmaderer, Christoph & Tauber, Robert & Brosch-Lenz, Julia & Haller, Bernhard & Meissner, Valentin & Knorr, Karina & Weber, Wolfgang & Eiber, Matthias. (2023). Journal of Nuclear Medicine. 65. jnumed.123.265986. 10.2967/jnumed.123.265986.
Safety and Efficacy of [177Lu]-PSMA-I&T Radioligand Therapy in Octogenarians with Metastatic Castration-Resistant Prostate Cancer: Report on 80 Patients over the Age of 80 Years. Tauber R, Knorr K, Retz M, Rauscher I, Grigorascu S, Hansen K, D'Alessandria C, Wester HJ, Gschwend J, Weber W, Eiber M, Langbein T. J Nucl Med. 2023 Aug;64(8):1244-1251. doi: 10.2967/jnumed.122.265259. Epub 2023 Jun 15. PMID: 37321824.
[1] Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy
Theranostics Center of Excellence
We are proud to announce that we have been accredited as a Theranostics Center of Excellence by the European Association of Nuclear Medicine (EANM). Currently, our facility is the only one in Austria to be awarded the EARL certificate.
Patient’s Testimonial: Full Remission after ¹⁷⁷Lu-PSMA therapy
Patient's Testimonial: Full Remission after ¹⁷⁷Lu-PSMA therapy
Here is a testimonial of one of our patients:
"I would like to announce hereby to the world that the small private clinic Minute Medical in Vienna led by Professor Markus Hartenbach, an expert in Nuclear Medicine, is nothing but a giant in helping people in need of medical attention, proving the success of day-to-day practical application of the results of the latest cutting edge scientific medical research.
Dr. Hartenbach had the courage to go against the tide of dusty, stone age “one-size-fits-all-protocols” in the treatment of metastatic prostate cancer, as a humble service to diseased men, who otherwise would have ended up going through depraving and for the human body harmful and often intolerable treatments that would result in the deterioration of the quality of life of people exposed to those treatments.
What are we talking about? The PSMA directed Lutetium-177 ligand therapy for metastatic prostate cancer, which is normally tough or impossible to access. According to my experience, most international doctors follow a “Protocol”, where affected people should rather be exposed to overall abdominal radiation, chemotherapy and years of chemical castration through hormone therapy, which often leads to the building of hormone-resistant cancer cells that cannot be treated any more and the patient is basically left to spend the rest of his life with the spreading cancer in his body. Not to mention the side effects caused by non-focussed radiation, by chemo, not to mention the hardly tolerable effects of hormone therapy.
Prof. Hartenbach is serving his patients by the motto of “individualized, tailor-made treatments”, meaning that he has the courage to apply the suitable most modern treatments at the right time to his patients. As is usual in a private clinic, however these are patients who are striving to receive this treatments at their own cost and possible peril, because they aim to maintain their lifestyle, their ability to enjoy life and their mobility despite their horrible disease, which, to my opinion, under normal “protocol” is made intolerable for the human body, slowly destroying everything in their lives that’s worth living for.
I’ve been writing this predominantly in third person till now, to give a somewhat objective report on the case. However, and unfortunately for me, I’m also subjected to this disease, and I went through a long process to find Prof. Hartenbach’s clinic and ask him to treat me. My name is Dr. Sandor Ambrus and I’m 70 years of age.
I can really call myself lucky to have been advised, and therefore being eternally grateful, to my wonderful and widely, as well as deeply, educated family doctor and General Practitioner who is up to date in the latest groundbreaking technologies, Dr. Andrea Szelenyi of Budapest, Hungary, who called my attention to the existence of this special therapy. I am also grateful to Prof. Stefan Förster who is heading up the Bayreuth University Clinic, who could not treat me because of the compulsory “Protocol” in public clinics, that is still controlling the medical practice in Germany. But he was kind enough to introduce me to Prof. Hartenbach and his team, who were able to “tailor-made” adapt the therapeutic approach according to my individual requirements in his above mentioned clinic “Minute Medical” to serve the ones in need with his top-notch treatment.
I had a drastic prostatectomy in January of this year (2024) in Budapest, Hungary, and was diagnosed after the surgery with further lymph nodes that were affected by metastases. I had a PSMA PET Scan that was showing the affected nodes. According to Hungarian “Protocol” – the same as the German one I was advised to go through the above-mentioned treatments, which filled me with a dread that induced sleepless nights, but even worse, horrible nightmares in the night to be awaken by the reality that was even more frightening than those scary dreams. This situation lasted till the moment I met Prof. Hartenbach who encouraged me to have the freedom of choice for my own body.
I had three Lutetium therapies in a row with a month between them. Each treatment took 15 minutes and a pleasant and educational conversation with Prof. Hartenbach regarding my health situation and the outlooks I may have. I have not experienced any side effects whatsoever then and since. After finishing the treatment, I had a repeated PSMA PET Scan that showed the complete remission of the cancer. This may obviously change with time, as you can never know with this disease, but I’m hoping that it would remain like this for a long time. In the worst case scenario that it reappears some time down the line, one can eventually repeat the treatment, depending on the circumstances at the time.
As for my current situation, I live my life to its full extent like before I was diagnosed with the disease. I’m as mobile as I have always been in the past fifty years, working in my business, having a beautiful family life and flying at least once a week within Europe and several times a year overseas. I’m driving tens of thousands of kms a year, and none of this has changed since I made up my mind to see Prof. Hartenbach.
Sincerely,
Dr. Sandor Z. Ambrus"
EANM24: The Age of Theranostics is NOW
The Age of Theranostics is NOW
You can listen to this article.
The European Association of Nuclear Medicine (EANM) hosted its annual conference, EANM24, from October 19-22, 2024, in Hamburg, Germany. EANM conferences are known for showcasing advancements in nuclear medicine, which can be broadly categorized into key areas:
- New Targets: Identification of novel proteins and enzymes in different cancers and lesions
- Novel Ligands: Development of transport molecules with enhanced binding capabilities
- New Tracers: better more potent radionuclides with a more limited radiation radius to minimize the damage to the surrounding healthy cells
- Expanded Applications of Known Radioligands: discovery of known proteins / enzymes in other cancers and lesions.
As usual during such professional get-togethers, this year’s conference also focused on how to bring theranostics to patients, addressing technical issues such as clinic setups, doctor training, patient flow, and enhancing the “last mile” delivery. Notably, one presentation from Finland examined the feasibility of outpatient theranostic treatment—something our clinic has been a shining example of. 1
Streamlining and unifying EU-wide regulations would further improve patient access to nuclear medicine therapies, with Austria providing a strong model for efficient practice.
AI and Nuclear Medicine
Artificial intelligence was a prominent topic, with discussions on AI’s potential to enhance nuclear medicine, for example, in interpreting PET scans. AI's ability to improve diagnostics could represent a substantial advancement in accuracy and efficiency.
Applications of PSMA- and DOTATATE-Based Theranostics
PSMA and DOTATATE-based imaging and therapy have become standard-of-care, with over a decade of clinical experience now guiding the development of new tracers and targets, such as FAP. A major theme was expanding PSMA therapy to earlier stages of prostate cancer and addressing resistance issues by shortening treatment intervals — a protocol our clinic has pioneered. Our streamlined protocol consists of three sessions with four-week intervals in contrast to the usual six to eight based on the research of our chief nuclear specialist Prof. Dr. Hartenbach. This regimen reduces cancer’s adaptive potential, yet still remaining tolerable for patients.
The revolutionary impact of PSMA PET/CT in clinical decision-making is being reaffirmed, with numerous studies corroborating its value in improving diagnosis and survival outcomes.2
New research on somatostatin receptors in myelomas and PSMA enzymes in gliomas holds promise for treating these cancers with targeted radionuclide therapy.3
New Targets
Approximately one-third of prostate cancers are PSMA-negative and do not respond to PSMA theranostics, requiring alternative markers. One study revealed high CD13 positivity in PSMA-negative prostate cancer, suggesting the protein CD13 as a viable target for theranostics.4
Emerging Radioligands and Radionuclides
New developments in radioligands include the DOTA-LM3, labelled with 161Terbium ([161Tb]Tb-DOTA-LM3), which demonstrated a seven-fold increase in tumor absorption compared to [177Lu]Lu-DOTATOC due to its superior two binding points.5
Terbium-161 as such also emerged as a promising radionuclide alternative to an often scarce Lutetium-177. 161Tb offers more concentrated radiation while maintaining favorable biodistribution. This radionuclide emits β- radiation similar to Lutetium-177, while also emitting therapeutically active very short range effective conversion electrons and auger electrons in combination with its low energy γ-ray emission, allowing for imaging via Single Photon Emission Computed Tomography (SPECT).6
Fibroblast Activation Proteins (FAPi)
FAPi warrants special attention. These proteins, characteristic of inflamed fibrous tissues, have been discovered in the microenvironment of most solid tumors, presenting a promising avenue for a universal therapy in stromal cancers. Stroma plays a critical role in cancer proliferation by nurturing tumors and shielding them from the body’s immune response. The oncological reality, however, is vastly more complex and heterogeneous. While FAPi-based imaging has shown success, therapeutic applications continue to face challenges related to suitable ligands.
These highlights only scratch the surface of the knowledge shared at EANM24. We will delve deeper into select topics and studies in upcoming publications, focusing on those most relevant to our patient cohort.
1 OP-093 “Establishing a 177Lu-PSMA treatment site in an oncology outpatient day-ward”, T. Noponen, A. Saikkonen, L. Kääriä, M. Seppänen, K. Mattila, A. Ålgars
2 OP-423 “PSMA-PET and PROMISE re-define stage and risk in prostate cancer patients”, M. Karpinski, J. Hüsing, K. Claassen, L. Möller, H. Kajüter, F. Oesterling, V. Grünwald, L. Umutlu, H. Lanzafame, T. Telli, A. Merkel-Jens, A. Hüsing, C. Kesch, K. Herrmann, A. Stang, B. Hadaschik, W. P. Fendle
3 OP-359 “Assessing the theranostic potential of SSTR imaging in advanced multiple myeloma patients - the SCARLET trial”, W. Delbart, I. Karfis, M. Vercruyssen, S. Vercauteren, Z. Wimana, N. Meuleman, P. Flamen, E. Woff
OP-514 “First-In-Human Experience of Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE in Patients with Advanced Multiple Myeloma”, W. Delbart, I. Karfis, M. Vercruyssen, S. Vercauteren, Z. Wimana, N. Meuleman, P. Flamen, E. Woff
EP-0113 “Diagnostic utility of 68Ga-Prostate-Specific Membrane Antigen-11 PET/CT in glioma recurrence - a prospective analysis”, A. Meena, K. Subramanian, R. Kumar, H. Singh, B. Mittal
EP-0653 “68Ga/177Lu-PSMA theranostics in recurrent high-grade glioma - First study results & future perspectives”, A. Karlberg, B. E. Vindstad, E. M. Berntsen, H. Johansen, T.M. Keil, O. Solheim, S. Kjærnes Øen, T. Skeidsvoll Solheim, L.Eikenes
4 OP-424 “CD13 as a Potential Membrane Marker in PSMA-Negative Prostate Cancer: A Complementary or Superior Alternative to PSMA”, Y. Tang, L. Xiao, J. Yang, J. Hou, J. Hong, A. Rominger, K. Shi, S. Hu
5 OP-252 “Therapy with the somatostatin receptor antagonist DOTA-LM3 labeled with terbium-161: Interim results of the Phase 0 Study in patients with gastroenteropancreatic neuroendocrine tumors”, J. Fricke, F. Westerbergh, L. McDougall, C. Favaretto, E. Christ, G. Nicolas, S. Geistlich, F. Borgna, M. Fani, P. Bernhardt, N. van der Meulen, C. Müller, R. Schibli, D. Wild
6 OP-515 “When Lutetium-177 DOTATATE Is Not Available: Insights Into Use of Terbium-161 in the Treatment of Metastatic Paraganglioma”, N. Jacobs, O. Kolade, K. Hlongwa, S. More
OP-529 “Mixed-LET 161Tb-ART-101 radiopharmaceutical enhances therapeutic responses in advanced prostate cancer”, M. Bio Idrissou, J. Tromp, H. Comas Rojas, L. Lambert, A.
Pinchuk, Y. Medina, A. Carston, R. Hernandez
OP-532 “Development of [161Tb]Tb-DOTA-HYNIC-panPSMA for targeted radionuclide therapy of prostate cancer”, C. Morgat, D. Vimont, K. Attia
UpFrontPSMA Trial Results: PSMA Therapy Advances to the Frontlines of Prostate Cancer Treatment
UpFrontPSMA Trial Results: PSMA Therapy Advances to the Frontlines of Prostate Cancer Treatment
The results of the UpFrontPSMA trial are in, unveiled during the ESMO 2024 in Barcelona, and they are truly encouraging for the future of prostate cancer treatment. 177Lu-PSMA radioligand therapy (RLT) has taken a significant leap forward in the treatment protocol, now positioning ahead of docetaxel chemotherapy. Initially introduced after the VISION trial as a last-in-line therapy for advanced metastatic castration-resistant prostate cancer in heavily pre-treated patients, this RLT has shown remarkable efficacy and a favorable safety profile, allowing it to move closer to the forefront of treatment options. In fact, it seems like the earlier, the more effective it is.
Trial Overview
The UpFrontPSMA phase 2 trial evaluated the efficacy of administering 177Lu-PSMA prior to docetaxel compared to docetaxel alone in patients newly diagnosed with high-volume metastatic hormone-sensitive prostate cancer (mHSPC). The experimental group received two cycles of 177Lu-PSMA (7.5 GBq each) followed by six cycles of docetaxel, while the control group received only docetaxel. The primary endpoint[1] was achieving undetectable prostate-specific antigen (PSA) levels after 48 weeks—a high bar for success.
Participant Selection Criteria
The most critical selection criterion for the trial was high PSMA uptake, with patients requiring a SUVmax above 20 based on 68Ga-PSMA PET/CT scans. (As researchers reported, some patients had to be excluded despite having initially demonstrated PSMA-avid lesions. This exclusion occurred because androgen deprivation therapy (ADT) prior to treatment led to downregulation of PSMA expression.)
Adverse Events
The combination of 177Lu-PSMA with docetaxel did not result in increased toxicity. The most common severe adverse events were the same in the two groups:
- febrile neutropenia (11% in the combination experimental group vs. 10% in the docetaxel-alone control group) and
- diarrhoea (6% in the combination group vs. none in the control group).
The only adverse event that was different in the experimental arm was dry mouth, a known common side effect of the 177Lu-PSMA. But even this one was all grade 1, so very mild.
Being a targeted therapy, 177Lu-PSMA was well tolerated with fewer side effects, resulting in better quality of life (QoL). Unlike docetaxel, which caused a rapid decline in QoL, patients receiving 177Lu-PSMA maintained their well-being longer, with QoL dipping only after the introduction of docetaxel.
Protocol Considerations
There were some concerns about whether the experimental arm might have been underdosed, as only two cycles of 177Lu-PSMA were administered. Typically, three cycles are standard practice. However, the trial's design aimed to avoid delaying conventional docetaxel treatment.
As a side note, our clinic follows a stricter protocol, spacing the cycles four weeks apart and not the usual six to eight based on the research of Prof. Dr. Hartenbach, our leading specialist. In general, German university hospitals, having played a pivotal role in developing PSMA-targeted therapies in the early 2000s, have accumulated significant expertise in this area.
Results
The results of the trial were highly promising. 41% of patients in the 177Lu-PSMA plus docetaxel group achieved undetectable PSA levels at the 48-week mark, compared to just 16% in the docetaxel-alone group. This significant difference demonstrates the superior antitumor activity of administering 177Lu-PSMA therapy before docetaxel, without an increase in toxicity. These findings promise to trigger a change in the standard-of-care and introduce 177Lu-PSMA in the treatment of mHSPC at the very early stage.
[1] The predefined, primary goal of a clinical study, against which its success is measured
WARMTH Act: ²²⁵Ac-PSMA in treating metastatic castration-resistant prostate cancer
WARMTH Act: ²²⁵Ac-PSMA in treating metastatic castration-resistant prostate cancer
The WARMTH[*]-initiated multicentre retrospective study investigated the efficacy and safety of 225Ac-PSMA radioligand therapy (RLT) in treating metastatic castration-resistant prostate cancer (mCRPC). This study included 488 men from Australia, India, Germany, and South Africa, aged between 37 and 90. Most patients had previously undergone multiple treatments, including chemotherapy, androgen-axis-receptor inhibitors, or 177Lu-PSMA RLT.
The findings show that 73% of the patients experienced some PSA decline after at least one cycle of 225Ac-PSMA RLT, with 57% showing a decline of 50% or more. Despite common side effects like xerostomia and bone marrow toxicities (with a notable prevalence of impaired bone marrow observed before treatment initiation), the therapy was generally well-tolerated, even in patients with pre-existing conditions.
With the study being retrospective, 225Ac-PSMA RLT was frequently administered as a last-line intervention when all other options had been exhausted and proven ineffective.
A significant association was found between a PSA decline of at least 50%, absence of prior exposure to specific treatments, lack of certain metastases, and absence of anaemia at the initiation of 225Ac-PSMA RLT with extended both progression-free and overall survival. However, the number of treatment cycles did not correlate with improved progression-free survival or overall survival.
In conclusion, 225Ac-PSMA RLT demonstrated significant antitumor effects in mCRPC, even in individuals with compromised bone marrow and renal function. The impact of previous treatment history and metastatic burden on time to death or disease progression suggests that patients with a comparatively lower disease burden may derive greater benefit from 225Ac-PSMA RLT.
Our clinic has an established practice of offering our patients with a corresponding disease profile a combination therapy of 1 + 2 (one 225Ac-PSMA + two 177Lu-PSMA cycles).
[*] WARMTH – World Association of Radiopharmaceutical & Molecular THerapy
| Age, years | |
| Mean | 68·1 (8·8) |
| Range | 37–90 |
| Countries | |
| Australia | 57 |
| India | 111 |
| Germany | 72 |
| South Africa | 248 |
| PSA at baseline, ng/mL | |
| Median (IQR) | 169·5 (34·6–519·8) |
| Previous treatment for mCRPC | |
| Docetaxel | 324 (66%) |
| Cabazitaxel | 103 (21%) |
| Abiraterone | 191 (39%) |
| Enzalutamide | 188 (39%) |
| 177Lu-PSMA RLT | 154 (32%) |
| Radium-223 dichloride | 18 (4%) |
| Pattern of disease | |
| Bone metastases | 435 (89%) |
| Lymph node metastases | 352 (72%) |
| Visceral metastases | 99 (20%) |
| Peritoneal metastases | 8 (2%) |
Transforming cancer from lethal to chronic. Therapy response after 2 cycles (6 sessions) of ¹⁷⁷Lu-PSMA therapy.
Transforming cancer from lethal to chronic. Therapy response after 2 cycles of ¹⁷⁷Lu-PSMA therapy
The case illustrates the scientific findings that the radioligand therapy 177Lu-PSMA is safe and effective and can be successfully applied more than once in case of recurrence, moving towards the goal of transforming cancer from lethal into a chronic disease like any other. It also grants the patients considerably better quality of life than the protracted ADT or chemotherapy.
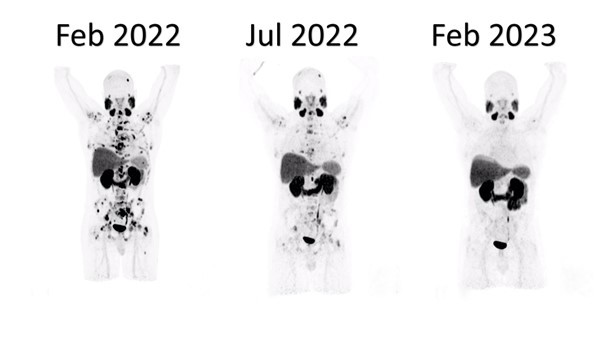
72 y.o. patient William Daly was first diagnosed with prostate cancer (PC) in December 2021. Despite heavy pre-treatment with several cycles of immunotherapy, cryoablation and leukin therapy, the disease continued to progress extending to lymph nodes. Mr. Daly refused constant antihormonal therapy and chemotherapy due to side effects and opted for 177Lu-PSMA targeted radioligand therapy. The patient has undergone 2 cycles 3 infusions each of the therapy. After the first one, PC went into remission for 1,5 years.
| BEFORE 1st cycle | AFTER 1st cycle |
| PSMA PET/CT October 2021
PSA 45 ng/ml. High PSMA-expressing lymph node metastases. |
PSMA PET/CT April 2022
PSA 0,18 ng/ml. After 3x 177Lu-PSMA therapy sessions from December 2021 to February 2022, significant remission of the retroperitoneal and locoregional lymph node metastases. PSA decline was documented until October 2022 with a Nadir of 2,39ng/ml. |
| BEFORE 2nd cycle | AFTER 2nd cycle |
| PSMA PET/CT September 2023
PSA 23,3ng/ml Progressive retroperitoneal lymph node disease with two new bone lesions as well as progression in the prostate. 3-session cycle of 177Lu-PSMA radioligand therapy is repeated 1,5 years after the first one. A short-term complimentary ADT with Relugolix was initiated along with the RLT to increase PSMA expression. |
PSMA PET/CT April 2024
PSA 0,1 ng/ml After 3x 177Lu-PSMA radioligand therapy sessions from December 2023 to February 2024 no activity in the metastases was detected in September 2023. The only side effect reported was short-term mild nausea and mild fatigue after the treatment. Meanwhile the patient stopped all medication and reports general well-being and regained strength. |
Read Mr. Daly's story in his own words:
"My story began in December 2014 when I was diagnosed with prostate cancer (PC). It was identified as “intermediate risk” with a Gleason score of 7 (3+4) in 3 of the 12 biopsy samples taken. At this point it had not spread, but after an MRI, it showed an “extracapsular extension”. I chose not to have surgery and decided to do watchful waiting. I also wanted to stay away from hormone therapy.
After another MRI, it showed that the PC was very near the seminal vesicles and I engaged a doctor in Fort Lauderdale, Florida to help me with treatment. He performed a 3D mapping biopsy that showed Gleason 8 in the prostate. Then he treated the PC with focal cryoablation, and my PSA went from 9 to 0.6. This was done in October 2017.
The PSA stayed there for about 2 years before it began to gradually move up. As it happens, I was treated for a kidney stone in July of 2020 and a swollen lymph node was noted in my pelvis area. After another MRI, my doctor in Florida suggested that we go through immune therapy. His immune therapy was very successful in some of his patients – but I did not respond to it. It was at that time that we pursued the 177Lu-PSMA treatment with Dr. Hartenbach. First, I had a PSMA scan done in Houston, Texas to see if the treatment could be done at all. After approval, I made arrangements to go through the therapy with Dr. Hartenbach. My PSA was about 48 at that time. That was in the fall of 2021.
After 1 cycle (3 infusions) of the therapy, my PSA was 2.39. The follow-up PSMA scan showed a remarkable improvement. There were only a couple of places where the cancer was still in the process of dying. We were very pleased at the response. This was in the spring of 2022 and the therapy had given me 2 years of remission. After closely monitoring the PC with scheduled labs, the PSA began to move up again. When it reached 23, we felt more treatment would be beneficial.
So, in December of 2023 I started another cycle of the 177Lu-PSMA therapy. We also added a drug called Orgovyx to the therapy. The PSMA scan was done in April after the treatment was completed in February 2024. I guess I can say that I experienced minimal side effects from the treatment (some nausea for a couple of days and some “dry mouth”). So, all in all, I was treated with 2 cycles (6 actual infusions) by Dr. Hartenbach.
This time, the results of the treatment and scan were remarkable. The PSMA scan was clear and the report said I had a “complete response” to the therapy – my PSA is now 0.1. This couldn’t have been a better result and I was so grateful and blessed to have been working with Dr. Hartenbach and his staff. I am praying for a long - term remission.
Thanks again for Dr. Hartenbach and all his staff."
Breast Cancer Breakthrough: The First Theranostic Treatment with DOTATATE. A Game Changer?

Breast Cancer Breakthrough: The First Theranostic Treatment with DOTATATE. A Game Changer?
Recent research has found that somatostatin receptor type 2 (SSTR2) is overexpressed in certain types of breast cancer, particularly in estrogen receptor (ER) positive tumors. This discovery paves the way for the potential use of the well-established DOTATATE theranostics to improve the diagnosis and treatment of this type of breast cancer. A study involving both preclinical and phase 2 clinical trials demonstrated highly promising results.
“This treatment has given me back my life.”
"This treatment has given me back my life."
Success story after 3 sessions of radioligand PSMA therapy with 1x Actinium-225 & 2x Lutetium-177
Sean Kenny was first diagnosed with prostate cancer in December 2020, when he just turned 51 y.o. Before landing in our clinic, he had undergone multiple treatments, including 6x Docetaxel, antihormonal therapy, immunotherapy and cryotherapy. He chose to pause ADT due to side effects but had to re-induct antihormonal therapy with Firmagon and Apalutamide mid-2023. The cancer was still progressing with an increasing bone involvement. Despite PSA response, pain and PSMA expression persisted. He was put on morphine medication for pain relief. His left hip was so badly damaged that it had to be replaced. After 3 years of back-and-forth Sean opted for PSMA targeted radioligand therapy (RLT) and experienced a remarkable recovery following just one 3-session cycle. Due to heavy bone infiltration, we recommended the first session be done with Actinium-225 and the two remaining - with Lutetium-177. Judge for yourself.
|
BEFORE PSMA PET/CT December 2023 PSA 2 ng/ml after re-induction antihormonal and ARPI treatment. St.p. hip replacement to the left previously. Still high PSMA expressing bone metastases and pain under morphine medication. |
AFTER PSMA PET/CT April 2024 After 1x 225Ac and 2x 177Lu PSMA ligand therapy sessions from December 2023 to March 2024. PSA 0,18 ng/ml. Minimal residual activity in the known bone lesions, most likely apoptotic cells. ALP down from 570U/l to 180U/l (norm <150). |
Sean experienced pain relief 10 days after the 1st RLT session with Actinium-225 PSMA and was able to get off the morphine medication. Hemoglobin and kidney values normalized after the 2nd RLT session with Lutetium-177 PSMA. The only side effect he experienced was short-term mild nausea and mild fatigue after the treatment. At the time-point of the control PSMA PET/CT, these symptoms were gone. Sean reports general well-being and regained strength.
Here is the story in his own words:
"My story started back in December of 2020 when I was told that I had advanced prostate cancer, which had spread to my lymphatic system. This was picked up with a routine blood test when I turned 50 years of age and when they included the PSA as a blood marker. I did not have any obvious symptoms at the time and in fact I was still running 7-minute miles and was very active.
I was put on hormone treatment in January of 2021 after I came back from the Royal Marsden hospital in the UK where I got a PSMA scan (I could not get a scan in Ireland for 9 months). Later in 2021 I started a course of 6 x Chemo (Docetaxel) and then 4 weeks of RT. In early 2022 I came off the hormone treatment due to side effects (my PSA has gone to a very low figure). I got my life back again as I regained my energy, and my fatigue was a lot less.
Then in early 2023 my PSA started to slowly rise again, so we looked into travelling to the US to undergo immunotherapy and cryotherapy at a clinic. While waiting for this clinic to admit me I developed an intermittent pain in my left leg which I relayed to my oncologist during an outpatient visit. After immediate X-rays of my leg, I was told that I had an impending fracture of my femur and needed an emergency hip replacement (my pelvic bone was also diseased). My hip was replaced 2 days later, and when I was strong enough again, I went to America to undergo this procedure in the hope that it would put me into remission again. Unfortunately, this operation was not successful, and within a month of coming home the pain I was suffering in my leg area was unbearable due to the metastasis in my pelvic area. I was put straight back on hormone treatment again and was told that I would be also receiving RT again.
My good Wife Brid had been in touch with the American clinic looking to see if there were other options available. This was then when I was referred to Prof Hartenbach and the rest as they say is history. I’m off all pain medication and with the hopes of coming off both my hormone treatments in the near future. From there it will be active surveillance and fingers crossed, but certainly this treatment has given me back my life.
Thank you, Prof. Hartenbach and team."
Harnessing Alpha Emitters in Radiopharmaceuticals for Cancer Treatment
Harnessing Alpha Emitters in Radiopharmaceuticals for Cancer Treatment
In the ever-evolving battle against cancer, the field of radiopharmaceuticals has emerged as a potent weapon, and alpha emitters such as actinium-225, astatine-211, and lead-212 are quickly gaining prominence. These alpha-emitting isotopes are revolutionizing cancer treatment, offering targeted therapy with the potential to eradicate malignant cells while minimizing damage to surrounding healthy tissue.
Reimagining Treatment Timelines: ¹⁷⁷Lu-PSMA at Early Stages Ahead of Conventional Therapies
Reimagining Treatment Timelines: ¹⁷⁷Lu-PSMA at Early Stages Ahead of Conventional Therapies
Radioligand therapy (RLT) utilizing 177Lu-PSMA gained initial approval in the USA as a final recourse once all other treatment options have been exhausted. However, quite a few ongoing trials are exploring the potential for deploying this therapy earlier in the course of the disease due to its demonstrated safety, efficacy, and relatively low incidence of side effects. In fact, radioligand therapy is very safe. A recent study has demonstrated that even 6 sessions are very well tolerated, what makes this treatment a viable option as a recurring therapy in case of recurrence.
¹⁷⁷Lu-DOTATATE in meningioma treatment
¹⁷⁷Lu-DOTATATE in meningioma treatment
Peptide receptor radionuclide therapy (PRRT) has significantly advanced in the last two decades within the realm of neuroendocrine tumours (NETs), leveraging the overexpression of somatostatin receptors characteristic of these tumours. This same receptor profile is also prevalent in nearly all meningiomas, with expression of somatostatin receptor 1 & 2 (SSTR1 / SSTR2), rendering PRRT a viable treatment option. Multiple studies have scrutinized the efficacy of PRRT (177Lu-DOTATATE) in meningioma patients, consistently demonstrating its effectiveness, particularly in prolonging progression-free survival (PFS).
⁶⁸Ga-PSMA PET/CT – unlocking precision

Unlocking precision: the revolutionary impact of ⁶⁸Ga-PSMA PET/CT in prostate cancer care
In the realm of medical diagnostics, the groundbreaking procedure of 68Ga-PSMA PET/CT has emerged as a game-changer, offering unprecedented insights into the assessment and management of prostate cancer. Its extraordinary precision enables clinicians to visualize prostate cancer at a molecular level. It has revolutionized the diagnosis, staging, and personalized treatment strategies for prostate cancer, ultimately enhancing patient outcomes and reshaping the landscape of prostate cancer care. Below is the review of just some of the latest studies.
Nuclear medicine – a versatile tool for treating many cancers
Nuclear medicine can help cancer patients live longer and enjoy a higher quality of life
Nuclear medicine is the fastest growing medical field, specifically in cancer treatment. It is extremely effective and low in side effects affording patients a prolonged and higher-quality life compared to conventional treatments like chemotherapy. It is also very versatile and applied in treatment of many cancer types. Our clinic not only provides cutting-edge therapies such as the radioligand PSMA therapy with 177Lu and 225Ac, as well as 177Lu-Dotatate therapy, but also offers the latest diagnostic procedure, 68Ga-FAPI PET/CT. Suitable especially well for such cancer types as breast, colon and pancreatic carcinomas, it represents a pioneering approach in diagnostic precision. Here you can learn more about FAPI in diagnostics.
¹⁷⁷Lu-PSMA is safe and effective for octogenarians
¹⁷⁷Lu-PSMA is safe and effective for octogenarians
A study evaluating the efficacy and safety of 177Lu-PSMA radioligand therapy for treating mCRPC in octogenarians demonstrated that radioligand therapy is safe and effective also in elderly patients. Moreover, patients who have not previously undergone chemotherapy had a better and longer-lasting response both in terms of overall and progression-free survival.
¹⁷⁷Lu-PSMA RLT and SBRT combined
¹⁷⁷Lu-PSMA RLT and SBRT combined
The challenge of the radiation therapy in cancer treatment lies in achieving an optimal radiation level within tumours and metastases, effectively eradicating cancer cells while minimizing harm to surrounding healthy tissues. The combination of Stereotactic Body RadioTherapy (SBRT) and internal radioligand therapy (RLT) with 177Lu-PSMA appears to be precisely the way to maximize the treatment of oligometastatic prostate cancer.
A recent study demonstrated a remarkable increase in the median biologically effective dose (BED) to 159 Gy with the combined 177Lu-PSMA RLT and SBRT. Importantly, this was achieved without significant side effects, whereas standalone external radiation typically falls short of reaching 70Gy. SBRT, strategically applied post-RLT, precisely targets any remaining PSMA-positive metastases, as verified by a control PSMA PET/CT after RLT.
In collaboration with our partners specializing in Cyberknife, SBRT and proton/C-Ion-therapy, our clinic offers these advanced therapies in a coordinated approach. We are committed to providing our patients with the cutting-edge oncological treatment, ensuring the highest standards of care and precision in their fight against cancer.
⁶⁸Ga-FAPI superior to ¹⁸F-FDG
⁶⁸Ga-FAPI superior to ¹⁸F-FDG in Advanced Metastatic Pancreatic Cancer
Cancer operates insidiously, shaping a distinct microenvironment even before the emergence of actual cancer cells. This inherent challenge makes crucial early detection difficult. Enter the groundbreaking 'tracer,' designed to detect the FAP alpha receptor on cancer-associated fibroblasts (CAF). This innovative approach unveils the early fingerprints of cancer, akin to fingerprint powder, facilitating early diagnosis that significantly enhances therapeutic decision-making, as evidenced by the latest study.
In another noteworthy study, 68Ga-FAPI PET/CT proves its superiority over the conventional 18F-FDG in oncological diagnostics, especially for detecting metastases and local malignancy recurrence in advanced metastatic pancreatic cancer. The scans exhibit higher lesion intensity (SUV), underscoring the effectiveness of FAPI in targeting the microenvironment of solid tumors prevalent in sarcomas, breast, colon, and pancreatic cancers. Precise diagnostics translates to improved therapy decisions. Our clinic offers 68Ga-FAPI; contact us for a consultation and appointment.
Explore further details on FAPI diagnostics here.
Terbium-161
Radioligand therapy with Terbium-161
Radioligand PSMA therapy with the lutetium isotope 177Lu has proven to be very effective against metastatic prostate cancer. Nevertheless, there is still a share of patients who do not respond to this treatment. A possible reason may be insufficient radiation dose delivered to the cancer cells so that they are able to survive. So scientists are now experimenting with another isotope terbium-161 (161Tb) that emits a wider range of energies, namely conversion and Auger electrons next to beta radiation. It has decay properties similar to 177-Lutetium, half-life of about 7 days, but higher destructive power due to co-emission of conversion and Auger electrons. Their short radiation range allows it to release all their power directly in the cancer cells with almost no damage to the neighbouring healthy cells.
PSMA PET/CT to assess the response to ARPI treatment (enzalutamide)
PSMA PET/CT to assess the response to Androgen Receptor Pathway Inhibitors (ARPI) treatment
PSMA PET/CT is the current mainstay diagnostic tool to stage prostate cancer, especially valuable if metastatic, and to decide on the right treatment (suitability of radioligand therapy 177Lu-PSMA). The newest study has established that it can also be used to evaluate the effectiveness of the treatment with Androgen Receptor Pathway Inhibitors (ARPIs).
Radiopharmaceutical therapy moving up to earlier disease stages
Radiopharmaceutical therapy moving up to earlier disease stages
PSMAfore trial by Novartis (Phase III trial of 177Lu-PSMA-617 in taxane-naïve patients with mCRPC) has not only met its primary endpoint of radiographic progression-free survival (rPFS) but has shown amazing results with the potential to change the clinical practice, as presented at 2023 ESMO conference. Radioligand therapy (RLT) with 177Lu-PSMA-617 in pre-chemo patients brought about the median rPFS of 12 months compared to only 5,6 months in the control group under standard care. The radioligand treatment is also better tolerated with fewer grade 3 adverse effects. The results seem to demonstrate – the earlier in the course of the disease the RLT is applied, the more effective it is. Our own clinical practice certainly corroborates this in relevant cases.
¹⁷⁷Lu-DOTATATE effective against G2 & G3 gastroenteropancreatic NETs
¹⁷⁷Lu-DOTATATE effective against G2 & G3 gastroenteropancreatic NETs
Although rare (orphan) and slow growing cancer type, some neuroendocrine tumors (NETs) are associated with rapid progression and poor prognosis. Original authorization for 177Lu-DOTATATE (Lutathera®) was based on the results of the NETTER-1 trial for inoperable midgut NETs. In the NETTER-2 trial 177Lu-DOTATATE has now also demonstrated its efficacy in patients with Grade 2 and 3 advanced gastroenteropancreatic NETs (GEP-NETs), and at that - as the first line treatment for newly diagnosed patients.
Sharing one of our success stories of complete remission
Sharing one of our success stories of complete remission
| Before the therapy | After 3 sessions of PSMA radioligand therapy | Control scan a year later |
FAPI diagnostics
FAPI diagnostics
Fibroblast activation protein (FAP) is overexpressed in the tumor microenvironment or stroma of over 90% of solid tumors, what makes it a promising target for both therapeutic and molecular imaging applications. FAP-targeting molecular imaging has been quickly gaining steam in cancer diagnostics. It is especially useful for tumors with a strong desmoplastic (forming fibrous tissue) reaction, such as breast, colon, and pancreatic cancers.
¹⁷⁷Lu-PSMA in taxane-naïve patients
Effectiveness of 177Lu-PSMA without prior chemotherapy
Since the end of the VISION trial that proved 177Lu-PSMA to be an effective treatment of metastasized prostate cancer, the next question arose – does it work even better if applied earlier in the course of the disease? An exciting study has now demonstrated that 177Lu-PSMA applied prior to chemotherapy is indeed more effective.
The metaanalysis was designed to evaluate the impact of prior taxane chemotherapy on response and survival in mCRPC patients after 177Lu-PSMA RLT.
It pooled together 13 studies comprising 2.068 patients. The results were:
- Taxane-naïve patients had 1,82 times better odds of biochemical response, i.e. 1,8 higher chances of no PSA rise.
- Taxane-naïve status was a predictor of both: significantly better progression-free survival (over 40%) and overall survival (over 46%).
Another clinical trial underway looks at the effectiveness of 177Lu-PSMA RLT in parallel with the anti-hormonal Androgen Deprivation Therapy. Results are expected in Q3 2024.
Personalized targeted radioligand therapy is complex and time-sensitive

Producing radioligands
Personalized targeted radioligand therapy is complex and time-sensitive
Manufacturing radioligands is an extremely complex and time-sensitive process, because radiopharmaceuticals need to be “delivered” directly to the cancer cells within days after being synthesized. This is what personalized targeted radionuclide therapy, a game-changer in oncology, is all about. Having our own laboratory and highly proficient radiochemists helps us serve our patients in-time and with the highest possible flexibility.
Hybrid imaging in prostate cancer diagnostics
Dual-Tracer PET-MRI-Derived Imaging Biomarkers for Prediction of Clinically Significant Prostate Cancer
Some prostate cancer cases will never develop metastases or any clinical symptoms and are defined as clinically insignificant. Accurate diagnosis able to differentiate between clinically significant and insignificant lesions leads to better disease management. The latest research of our working group has demonstrated that combined hybrid imaging using [18F] fluoromethylcholine (FMC) PET and [68Ga]Ga-PSMAHBED-CC conjugate 11 (PSMA)-PET achieved higher sensitivity for detecting clinically significant prostate cancer compared to multiparametric MRI alone. The PSMA PET is the leading method in this hybrid approach and is in fact more reliable than MRI alone.
Clinic’s statistics on RLT ¹⁷⁷Lu-PSMA
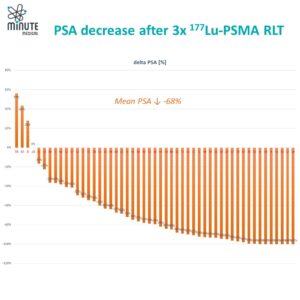
As many trials with 177Lu-PSMA radioligand therapy (RLT) have demonstrated, a reduction of PSA of over 50% leads to statistically significant increase in overall survival. Our clinic’s results show the reproducibility of this data. After 3 sessions of 177Lu-PSMA PSA decreases by an average of 68%. Preserving remissions and increasing quality of life of our patients remain our goals.
Pluvicto® approval by EMA
Pluvicto® approval by EMA
Following FDA approval in March 2022, European Medicines Agency (EMA) also approved Pluvicto® (lutetium (177Lu) vipivotide tetraxetan) of Novartis in December 2022 for targeted radioligand treatment of progressive PSMA–positive metastatic castration-resistant prostate cancer. The EMA approval is based on the results from the Phase III VISION trial, in which “radioligand therapy with 177Lu-PSMA-617 prolonged radiographic progression-free survival and overall survival when added to standard care in patients with advanced PSMA-positive metastatic castration-resistant prostate cancer”[1]. Pluvicto® is to be used together with androgen deprivation therapy in adults previously treated with androgen receptor pathway inhibitors and taxanes. “The lutetium-177-PSMA was developed by the German Cancer Research Center (DKFZ) in cooperation with Heidelberg University Hospital and Heidelberg University.”[2]
[1] https://www.nejm.org/doi/full/10.1056/nejmoa2107322
[2] https://www.krebsinformationsdienst.de/aktuelles/2022/news059-metastasierter-prostatakrebs-zulassung-pluvicto-lutetium-177-psma-617-europa.php
Prof.Dr. Haug awarded The European Awards in Medicine in the field of nuclear medicine
The European Award in Nuclear Medicine 2022
We are thrilled and proud to share that our colleague Prof.Dr. Alexander Haug has been awarded The European Awards in Medicine 2022 in the field of nuclear medicine.
Interview with a patient
Interview with a patient, September 2022
Dr. Hartenbach conducts an interview with a patient about his experience with the radioligand therapy 177Lu-PSMA. The patient A.G., 72 y.o., a molecular biologist himself, was diagnosed with metastatic prostate cancer in September 2018. After the conventional standard-of-care treatment (chemo- and antihormonal therapy) there were still residues of the primary tumour in the prostate, and lymph nodes and bone metastases. Up to date he has gone through 9 sessions (3 cycles) of 177Lu-PSMA therapy in the course of three years, initiating a new cycle once a routine control 68Ga-PSMA PET/CT detected PSMA expressing cancer lesions. The therapy has been extremely successful for A.G.: high response and hardly any side effects. Targeted radioligand therapy brings us much closer to the goal of making prostate cancer just another treatable chronic disease, at the same time preserving a decent quality of life, much like e.g. diabetes.
- [00:45] Can you tell us about your prostate cancer diagnosis and the first steps you took? How did you perceive the limitations of standard-of-care therapies and what made you look for alternatives?
- [06:13] How would you compare the side effects and effectiveness of radioligand therapy to standard-of-care treatments?
- [10:07] What is your personal experience with side effects like xerostomia or kidney issues after multiple radioligand therapy cycles?
- [12:00] The importance of the combined treatment approach, including hormonal therapy and carbon ion radiation.
- [15:44] What has helped you personally to cope with the disease and stay optimistic about the future?
Prof. Markus Hartenbach (MH): Hello and welcome to Minute Medical. My name is Markus Hartenbach and today we have a special guest here who's willing to give an interview. Professor Dr. Alexander Von Gabain, renowned microbiologist, founder, entrepreneur and much more. And we really appreciate that he's giving that interview today. Hello, Alexander.
A.G.: Hello, Markus. I'm looking forward to this interview and it's a pleasure for me to support you with what I have learned during the course of my disease and its treatments.
MH: Yes, we really appreciate that, Alexander. So you are not here as an analytical scientist. You are more here now because four years, pretty much exactly 4 years ago, there was a diagnosis that you were confronted with and maybe you can talk a little bit about what it was and what were the first steps.
A.G.: Well, I have been diagnosed indeed four years ago with an advanced prostate cancer with metastases in bones and in a number of lymph nodes. And being a molecular biologist and having worked by myself in oncology, I was aware that this will change my life upside down. It is a shocking experience and in the beginning you have life fear, and as a result, you lean on colleagues who are established and renowned. And I have to say, I was getting good treatment in a university hospital. The dilemma has been that the doctors, maybe also because I am coming from the field, they rather were inclined to implement for me the standard-of-care treatments. The standard-of-care treatments is of course suppressing of testosterone, nicely named by our colleagues as castration or chemical or really physical castration or also in sequence or together with a chemotherapy. And I have gone through this and there was some achievement.
But in honesty, you could still see in my body spreads of the original tumor and also the original tumor was still in the prostate. So this was quite a learning curve. And I have to say what I have learned from this curve is standard of care therapy is important and it's good. But as soon as you are breathing out and you see it's my cancer and it's my life, you have to see what else is on the way. And I was lucky to learn by accident, but also by systematically asking around in the circles of my colleagues and friends, that there are already the next generation treatments coming up. And I always see it through the eye classes of a molecular biologist. The chemotherapy and the hormone suppression therapy I have gone through was clearly still a medicine of the 20th century, but not of the 21st century. Why? I mean, they clearly hit the tumor, but they are far from being specific.
Take chemotherapy. Of course it's blocking most fast-growing cells, so it will block the cancer, but it also has partially very severe side effects on other tissue in your body which is dividing based on dividing cells.
Suppressing the hormones in itself is OK, but I think it's not specific. It also is taking away the testosterone for other functions where they are needed in your body for natural function.
So this learning curve was basically the kickoff point to find other therapies. And interesting, I came across and this is maybe a good thing about the prostate cancer. It has the most specific diagnostic method to discover tumor or tumor metastases in the body, namely by a specific molecule which binds to a surface structure of prostate cancer cells. And if you add the label on, for example, a radio label, you can identify wherever in the body there are still spreads of your cancer. And while I was going through this procedure several times and seeing that the treatments in part improved my conditions. I said, gee, if this is so specific to discover specific residual cancer cells in your body, there must be also a therapy which is based on this specificity. And that's when I met Markus and it wasn't quite an accident.
I heard about him through many sources and Markus explained to me that science has not been resting and I think they have been making this key molecule which is binding so to say to these receptors on the surface of prostate cancer cells by adding something on, you call this a magic bullet, which gives tougher radiation. But compared to the other treatments I have gone through, it is very, very specific. It only destroys cells where you have cancer cells which bind to the ligand and the ligand in the backpack has this radioisotope which is destroying the cancer cells. So that's just the story which I would say to give in short. But now we should not go into the depths.
MH: Yes, this is our theranostics approach which is providing a diagnostic tool with a PSMA ligand PET CT and then of course the therapy with lutetium- or actinium-labeled PSMA which is directly targeting the cells. This is what you what you mentioned. Maybe you can also give an outlook on the comparison between the standard-of-care therapies you had which were successful lowering PSA also killing metastasis in your body. But from the side effect profile, you have now undergone 9 sessions of lutetium PSMA therapy over the past three years, and the last therapy you had in April 2021, which is now 1 1/2 year ago. So also in case of long-term side effects, because now you are 1 1/2 year after your last of nine sessions, maybe you can give a little outlook and comparison of these therapies.
A.G.: Yeah, before I come to this, let me stay one more time with the standard-of-care treatments I have gone through. They indeed had, I would say, nearly unbearable side effects. I have to be honest, at some time during my chemo it I was even so exhausted I could not even go downhill. So not to talk about uphill. so but if you explain this as a molecular biologist, it's clear because you are hitting so many innocent body cells, I think, which so to say, lead to your bad conditions.
So this method just described by Markus has this high specificity. And I think this is maybe the amazing thing is that um the follow-up treatment which was proposed to me by my colleagues in this academic hospital was surgery plus radiation with radio beams and they were trying to explain to me that still see several herds of cancer cells in my body that could with two methods so to say to improve my condition. And I in this situation, facing further unspecific treatments because surgery as well radiotherapy can never so precisely hit the tumor herds in the body, as I think this just described method which I have gone nine times through and I have to say, to give the plain answer, I nearly did not have any severe side effects. As a matter of fact, I was still functioning. Markus is my doctor, also my coach sometimes. He encouraged me to continue my very active professional life. So I was not, so to say, calling off my board seats in a variety of interesting academic institution and companies. And I could do this even while I was getting this treatment. And I think this for me was a hallelujah experience because I started to move into molecular biology, I think by now, nearly more than 45, 48 years ago. And I think my first teachers belonged to the pioneers who have been deciphering the DNA and finding the first protein-protein interactions and explaining how cells are functioning. And I said gee, this knowledge has now really reached the patient. It really protects you in that it really kills only and mostly cells which are really affected by the cancer or cancerous cells. There are some other tissues in the body which also are a challenge. I think this is clear, but I have to say they have not given me any handicap. And I have to say so far I can say the only real measurable side effect that you are a bit tired. But I think any type of treatment gives you a tiredness, even if you're taking antibiotics.
MH: Maybe we can focus a little bit on that because most of the patients are afraid of the described and known side effects of radioligand therapy concerning dry mouth, xerostomia, and sometimes you have the feeling that it's a little bit exaggerated in the literature about xerostomia and dry mouth. What is your experience on that after so many cycles? In the literature we only have up to six cycles described. And we have treated patients over the past decade and never seen here in Vienna real xerostomia, real dry mouth also concerning the kidneys because it is in the kidneys, the PSMA complex, these are the known and described organs also with PSMA expression. What is your experience on that?
A.G.: My experience is that I have not really suffered from, I think, the condition of dry mouth until now, nor any problems in that regard. My kidneys, they show some weaknesses, but I'm convinced they come from other treatments that I've been taking, which would be a little too long here to explain this in detail. But I can say that the real well feeling and being during the therapy and after the therapy is so residual that you can almost lead even during the therapy time, not to talk afterwards anyway, you are not impeded in functioning in your private and also in your professional life. And I think this is very important because if you have cancer, this is a very important part of life quality I think that you continue with your normal life as much as possible.
MH: And what was also very important in your approach was the combined approach. And we always want to point out that not only the radioligand therapy is a standalone therapy. In your case you started with the conventional approach. We also had to the combination over all the time with the antihormonal, also third generation antihormonal treatment. And as a last treatment we also had the carbon ion targeted radiation which is also very precise. So in the end, I think this combination was the key to success.
A.G.: Yes, and by that I like to encourage, so to say, people who come into the same severe life situation as I have been, so to say, sliding in. If you take the choice of new treatments, it's also a gain in time. And by the time before I did the radioligand therapy, there were only, as Markus has mentioned, the 1st and 2nd generation, you can say, hormone receptor blocking treatments, so to say, in the medical community. And I think some of them only you can take by taking at the same time 50 milligram of cortisone. I mean they have, they have and they had really strong side effects and I think the time gain I had with this treatment made me luckily coming into so to say qualifying for the third generation of testosterone receptor blocking agents. And I have to say I take this now as an adjuvanted treatment. And again, I say it has something to do with molecular biology. Scientists have not been sleeping. The third generation, I think, apolutamide or darolutamide, they are much more specific than the first two generations. And so they are also for me with side effects, much more tolerable. And what Markus has mentioned too, I had and I keep my fingers crossed it was still like this, still some residual tumor herds in my prostate. And we thought that it would be good also to use a technology which is really bombarding my prostate but saving as much as possible the surrounding tissue like the rectum and the urine bladder. And this is with this carbon neuron radiation. And it is the same. I mean this is now not molecular biology, but I would say it is the chemical or physical chemical specificity of the nature of the radiation is a great improvement for that type of disease because you hit the prostate much more specifically than with the classical treatments with using X-ray radiation where you almost normally also harm a bit your urine bladder and I think often very strong in your rectum.
MH: Yeah, in your case it worked greatly, and I think in many other patients as well. The data is also showing that and of course you were always very well informed, also on a scientific level. But I also want to encourage the patients, ask for different therapies, for next generation therapies and only the patient that is well informed is the patient that will receive then the best treatment. As a last question and maybe then also as an outlook, it's more a personal question. I mean what was your secret, your personal secret, how to deal with it, your disease, in the past and now also for the future? And what is it that is making you look positively into the future.
A.G.: Well, I have to say I look into the future, and I look forward, and I'm aware with my disease this needs also to some degree good luck. But I guess anyway the most important thing is that this learning curve and being guided that specific interactions of the treatment agents and the treated cells have been really entering the field and improving the treatment. And what Markus just has been stating, there's an interesting study out which is saying, and this is a good thought experiment, if you overnight would take in let's say the societies which can afford this the next generation treatments, not only for prostate cancer, also for diabetes or whatever, you would not only save the healthcare systems of the societies a lot of money, but you would prolong life substantially of the people who are affected by those diseases. So it is a calculation which has been done. And the dilemma is that often through the back door, the next generation treatments are so to say coming in. But the standard-of-care is sometimes also a bit like, I would say, an impediment or almost like a prison for the thoughts. For the doctors, for the scientists, but also for the patients. And I think what I have seen in my case, taking the specificity as a guideline (I am, of course, privileged, I can understand this) I saw more and more at least I have a path which can protect me as long as it is possible. And that really gives me a lot of strengths and I think of course not only to talk about science and molecules and my treatment, as I should say, I have a family which supports me strongly and I have also got strong support by Doctor Hartenbach. I should say in this instance I call him not only a friend but also a coach in making the right decision. Also in my personal life, encouraging me to continue to lead a normal life.
MH: Very nice words. Thank you very much Alexander for your time and maybe for the future and for next generation therapy, we also have our fibroblast-targeted imaging and possibly therapy for the future. So the story is going on and life is going on. And thanks a lot for all these words and your time.
A.G.: It was a pleasure.
Manufacturing personalized radioligands in the laboratory
Manufacturing personalized radioligands in the laboratory
FAPi-based targeted theranostics for treating radioiodine-refractory differentiated thyroid cancer
FAPi-based targeted theranostics for treating radioiodine-refractory differentiated thyroid cancer
The study of FAPi-based lutetium-177 therapy in the All India Institute Of Medical Sciences in New Delhi was meant to investigate the therapeutic efficacy and safety of 177Lu-DOTAGA.(SA.FAPi)2 in RR-DTC patients. 15 heavily pre-treated (min 2 lines of prior treatment with lenvatinib and/or sorafenib) patients with the disease progression were selected based on FAPi PET/CT. An average of 3 sessions of 2 GBq each was administered in an 8-week interval.
TheraP study comparing radioligand treatment with ¹⁷⁷Lu-PSMA to the chemotherapy using cabazitaxel
Trial comparing radioligand treatment with ¹⁷⁷Lu-PSMA-617 to the chemotherapy using cabazitaxel in treating mCRPC
TheraP, a phase II trial comparing radioligand treatment with 177Lu-PSMA-617 to the chemotherapy using cabazitaxel was finalized with 177Lu-PSMA demonstrating a clear advantage over the chemotherapy.
EAU updates its Prostate Cancer Guidelines
EAU updates its Prostate Cancer Guidelines
After the conclusion of the Vision trials the urological associations start gradually adjusting their Prostate Cancer guidelines, both in diagnostics and treatment. EAU now strongly recommends:
- PSMA PET/CT (with gallium-68 or fluorine-18) as a more accurate diagnostic method than CT and bone scan for the staging of high-risk prostate cancer (PSA > 20 ng/mL, or GS > 7, or clinically palpable tumour in both lobes (cT2c)), and
- 177Lu-PSMA as a therapy of choice for pre-treated (ADT) mCRPC patients with one or more metastatic lesions, highly expressing PSMA on the diagnostic radiolabelled PSMA PET/CT scan.
https://uroweb.org/guidelines/prostate-cancer/summary-of-changes
Both are available in our institution. Make your appointment now.
FAPI
Nuclear theranostics: what’s next?
While theranostics has immensely improved overall survival and quality of life of NET and prostate cancer patients and is increasingly becoming mainstream, the science is tirelessly working on the next big thing – pan-cancer theranostics based on fibroblast activation protein (FAP) found in the cancer-associated fibroblasts (CAF). With many widespread cancers in question, this would be truly revolutionary. First-in-human PET/CT studies of 68Ga-FAPI have demonstrated excellent results. A tracer derivative better suited for the therapeutical ends is within reach.
Bone metastases, prostate cancer and theranostics
Bone metastases, prostate cancer and theranostics
Bone Metastases: A Common Secondary Cancer in Multiple Tumor Types
Bone metastases are a frequent complication in many cancer types, including breast (70%), prostate (85%), lung, and kidney cancers (40%), due to the unique characteristics of the bone microenvironment. Tumors predominantly metastasize to the axial skeleton—bones of the trunk and pelvis—rather than to the appendicular skeleton (limbs and girdles), following the distribution of red bone marrow.
Ga-68 demonstrates superiority to F-18 in digital PSMA-PET/CT scans for staging prostate cancer
Gallium-68 has demonstrated superiority to Fluorine-18 in digital PSMA-PET/CT scans for staging prostate cancer
Fluorine-18 is a commonly used isotope utilized for conducting PSMA-PET/CT to stage prostate cancer, due to its longer half-life and higher production capacity as compared to Gallium-68. The latest study, however, has demonstrated superiority of Ga-68, because of the focal unspecific uptake of F-18 in the bones of ribs and pelvis. Without additional follow-up exams or any morphological correlates, this may be misinterpreted as bone metastasis. While nonspecific uptake in other tissues and physiologic uptake in the ganglia can be filtered out, unspecific bone uptake tends to lead to false interpretation and misdiagnosis. Bone metastases do occur in 10% of patients with the newly diagnosed prostate cancer, and 80-90% of patients in the advanced stage. Over-staging the patient may result in inadequate therapy decisions, especially in case of early biochemical recurrence. Read more.
PSMA therapy under more restrictive regimen of 4-week intervals
PSMA therapy under more restrictive regimen of 4-week intervals
How oft is the Lu-PSMA treatment conducted? The usual cycle consists of 3 sessions with 6 to 8 weeks interval in-between. Our more restrictive treatment regimen of 3 initial cycles with an interval of only 4 weeks in-between demonstrated >80% response rates even in heavily pre-treated patients. This treatment does not care, “where” the metastases are: lymph nodes, bone, lungs, liver or even tumor in the prostate region itself. The usual “victims” of PSMA ligands, the salivary glands and kidneys, are not significantly affected under this regimen. Read more here.
Check other scientific investigations of our team on the topic.
PSMA therapy improves quality of life in prostate cancer patients
¹⁷⁷Lu-PSMA therapy improves health-related quality of life in patients with mCRPC
On Friday, 17 September 2021, Novartis announced further positive findings from the VISION trial: 177Lu-PSMA-617 therapy delays worsening of physical functioning and the onset of pain symptoms in patients with mCRPC. Hence, beyond extending overall survival and progress-free period, this Breakthrough Therapy also improves health-related quality of life. Read more
NCCN Guidelines add PSMA-PET imaging for prostate cancer
National Comprehensive Cancer Network, an alliance of cancer centers in the US, whose guidelines in oncology are applied to treating cancers, has added 68Ga-PSMA-PET imaging to its clinical practice guidelines for prostate cancer. Moreover, it recognized the high precision of this diagnostic tool as a primary stand-alone method and scrapped conventional imaging as a necessary prerequisite for PSMA-PET. Read more in the Urology Times.
Results of Phase III Vision Trial announced
On 23 March 2021 Novartis announced positive results of the phase III trial with radioligand therapy 177Lu-PSMA-617 in patients with castrate-resistant metastatic prostate cancer (mCRPC) after taxane chemotherapy and several androgen deprivation therapies (ADT).
Frontiers in Urology
Frontiers in Urology
Working group under Prof. Hartenbach shows outstanding diagnostic value of PSMA PET/MRI in prostate cancer
Working group headed by Prof. Markus Hartenbach was able to demonstrate the outstanding diagnostic value of PSMA PET / MRI in biopsy-proven prostate cancer. PSMA-PET/MRI can provide an accurate staging of newly diagnosed prostate cancer. In addition, treatment strategies were changed in almost a third of the patients due to the information of this hybrid imaging technique. The scientific paper was published in the Clinical Cancer Research journal.
Working group under Prof. Hartenbach MD demonstrates superiority of PSMA PET in diagnosing recurring prostate cancer
The working group headed by Prof. Markus Hartenbach MD was able to demonstrate the superiority of PSMA PET in the diagnosis of biochemical recurrence of prostate cancer, even at low PSA levels. Moreover, this diagnostic tool adds significant information to standard CT/MRI, changing treatment strategies in a significant number of patients. PSMA-positive lesions were detected in 85.5% patients, whereas 57.3% had no suspicious correlates according to the MRI or CT reports. Detection rates were 65% for a PSA value of 0.2 to <0.5 ng/ml, 85.7% for 0.5 to <1, 85.7% for 1 to <2 and 100% for ≥2. PSMA-PET changed therapeutic decisions in 74.6% of the patients (p < 0.001), with 86% of them being considered for metastases-directed therapies. The scientific paper was published in the “European Journal of Nuclear Medicine and Molecular Imaging”.
Markus Hartenbach wins Oncology Council’s Young Investigator Award
Markus Hartenbach wins Oncology Council's Young Investigator Award of the North American Association of Nuclear Medicine.