Harnessing Alpha Emitters in Radiopharmaceuticals for Cancer Treatment
Harnessing Alpha Emitters in Radiopharmaceuticals for Cancer Treatment
In the ever-evolving battle against cancer, the field of radiopharmaceuticals has emerged as a potent weapon, and alpha emitters such as actinium-225, astatine-211, and lead-212 are quickly gaining prominence. These alpha-emitting isotopes are revolutionizing cancer treatment, offering targeted therapy with the potential to eradicate malignant cells while minimizing damage to surrounding healthy tissue.
Alpha Emitters: A Brief Overview
Alpha particles are highly energetic, consisting of two protons and two neutrons. Their high energy and short range (a few cell diameters) make them ideal for destroying cancer cells with precision. These characteristics allow it to cause a double-strand break in DNA, what deals a final blow to the cancer cells, at the same time minimizing the collateral damage to nearby healthy cells. The alpha emitter will not be displacing beta emitters like lutetium but rather complementing them, as you will see below on the example of 225Ac employed in treating prostate cancer.
Actinium-225: Precision in Action
Actinium-225 is at the forefront of alpha emitter research for cancer treatment. With a half-life of appr. 10 days, Actinium-225-labeled radiopharmaceuticals have shown remarkable efficacy in treating metastatic cancers, particularly prostate cancer. Clinical trials have demonstrated significant tumour reduction and prolonged survival rates in patients with advanced-stage prostate cancer.
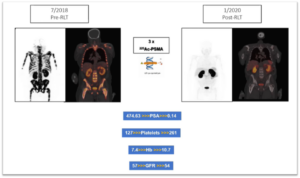
225Ac-PSMA in prostate cancer treatment
Especially in case of bone marrow infiltration of metastasized prostate cancer, alpha emitters can work in perfect combination with lutetium. The first session of radioligand therapy conducted with a more potent alpha emitter (e.g. 225Ac-PSMA) allows to destroy cancer cells in the bone marrow without affecting the marrow itself. This permits the body to recover its haematopoiesis, i.e. blood cell production, function. The study in South Africa was the first to demonstrate it. A patient with severe metastatic prostate cancer and heavy diffused bone infiltration recovered from severe anaemia after 2 cycles of 225Ac-PSMA. Haemoglobin values and platelets count improved dramatically, what obviously leads to the revival of the innate immune response of the body.
We have an established practice of offering our patients with a corresponding disease profile a combination therapy of 1 + 2 (one 225Ac-PSMA + two 177Lu-PSMA cycles).
225Ac-DOTATATE in NETs & meningioma treatment
Treating prostate cancer with radiopharmaceuticals is now considered a low-hanging fruit due to its well-established efficacy. Meanwhile, the versatility of actinium-225 allows it to be conjugated with various targeting molecules, expanding its potential applications to other cancer types, such as, neuroendocrine tumours (NETs) and meningiomas.
Using 177Lu-DOTATATE (with the beta emitter Lutetium-177) in the treatment of NETs in fact predates the use of 177Lu-PSMA for prostate cancer therapy. However, tumours can sometimes become resistant to lower-LET (Linear Energy Transfer) radiation therapies, such as those using beta emitters. Additionally, some aggressive or advanced forms may not respond adequately to beta-emitting therapies. In such cases, alpha emitters fare better due to their greater cytotoxicity.
Identifying DOTATATE receptors within meningioma cells, a prevalent type of brain tumour, has been another very recent breakthrough. Given the intricate nature of the brain, where critical structures tightly envelop tumours, accurate tumour targeting with minimal radiation exposure to healthy brain tissue becomes paramount to mitigate adverse effects. Alpha emitters, with their highly concentrated but constrained power, are an ideal choice.
225Ac-DOTATATE is available in our clinic.
Astatine-211: A Novel Approach
Astatine-211 is another promising alpha emitter, characterized by its favourable half-life (7.2 hours) and potent alpha emissions. Its short half-life ensures that the radioactive decay occurs primarily within the tumour, minimizing long-term radiation exposure to the patient.
Astatine-211 is being explored in the treatment of gliomas, a type of brain cancer with limited treatment options. Researchers have developed astatine-211-labeled monoclonal antibodies that specifically target tumour-associated antigens. Early-phase clinical trials indicate that this approach can deliver a high radiation dose to the tumour while sparing normal brain tissue, offering hope for improved outcomes in glioma patients.
Lead-212: A Dual-Mode Therapy
Lead-212 stands out due to its unique decay properties, emitting both alpha and beta particles. This dual-mode emission can be advantageous in certain therapeutic contexts. The alpha particles provide high-energy, short-range cytotoxicity, while the beta particles offer a more extended range, potentially targeting micro-metastases.
212Pb-DOTAMTATE has recently been granted “breakthrough therapy” designation by the FDA for the treatment of patients with advanced SSTR-expressing gastroenteropancreatic neuroendocrine tumors (GEP-NETs). This “designation of 212Pb-DOTAMTATE, an SSTR-targeting peptide complete radiolabeled with lead-212 generating alpha particles, is supported by phase 1 and ongoing phase 2 clinical trials (NCT05153772).”[2]
Lead-212 is also being investigated for use in treating ovarian cancer. The radiopharmaceutical 212Pb-TCMC-trastuzumab targets the HER2 receptor, overexpressed in some ovarian cancers. Preclinical studies have shown that this compound can effectively reduce tumour burden and improve survival in animal models. Clinical trials are underway to evaluate its safety and efficacy in human patients.
Managing Toxicities
While alpha emitters offer precision targeting, their high energy can also pose risks if not adequately controlled. For example, in treating prostate cancer too much of 225Ac-PSMA tends to damage the salivary glands (they also express the PSMA enzymes just as the prostate cancer cells) causing severe xerostomia (mouth dryness) with the side effects outweighing the benefits. One initial cycle of 225Ac-PSMA is often already sufficient to bring about the desired effect, while preserving the salivary glands.
The Future of Alpha Emitters in Cancer Therapy
The future of alpha emitters in cancer therapy looks promising, with ongoing research and clinical trials paving the way for new treatment paradigms. The integration of advanced imaging techniques, such as PET and SPECT, allows for real-time monitoring of radiopharmaceutical distribution and therapeutic response. This feedback can be used to adjust treatment plans and improve outcomes.
Additionally, personalized medicine approaches are being explored, where the specific genetic and molecular characteristics of a patient's tumour are used to tailor radiopharmaceutical therapy. This precision medicine approach has the potential to further enhance the efficacy and safety of alpha emitter therapies.
Conclusion
Alpha emitters like actinium-225, astatine-211, and lead-212 represent a significant advancement in the field of radiopharmaceuticals for cancer treatment. Their ability to deliver highly targeted, lethal doses of radiation to cancer cells offers a powerful tool in the fight against malignancies. As research progresses and challenges are addressed, these alpha-emitting radiopharmaceuticals hold the promise of improving survival and quality of life for cancer patients worldwide.
[1] [225Ac]Ac-PSMA-617 α-radioligand therapy of patients with extensive skeletal metastases of castration-resistant prostate cancer, Sathekge et al. 2022
[2] "FDA Grants Breakthrough Therapy Designation to Novel Agent in GEP-NETs"
Bibliography:
Matthew R. Griffiths, David A. Pattison, Melissa Latter, Kevin Kuan, Stephen Taylor, William Tieu, Thomas Kryza, Danielle Meyrick, Boon Quan Lee, Aaron Hansen, Stephen E. Rose, Simon G. Puttick First-in-Human 212Pb-PSMA–Targeted α-Therapy SPECT/CT Imaging in a Patient with Metastatic Castration-Resistant Prostate Cancer Journal of Nuclear Medicine Feb 2024, jnumed.123.267189; DOI: 10.2967/jnumed.123.267189
Albertsson P, Bäck T, Bergmark K, Hallqvist A, Johansson M, Aneheim E, Lindegren S, Timperanza C, Smerud K, Palm S. Astatine-211 based radionuclide therapy: Current clinical trial landscape. Front Med (Lausanne). 2023 Jan 6;9:1076210. doi: 10.3389/fmed.2022.1076210. PMID: 36687417; PMCID: PMC9859440.
Lawal IO, Morgenstern A, Vorster M, Knoesen O, Mahapane J, Hlongwa KN, Maserumule LC, Ndlovu H, Reed JD, Popoola GO, Mokoala KMG, Mdlophane A, Bruchertseifer F, Sathekge MM. Hematologic toxicity profile and efficacy of [225Ac]Ac-PSMA-617 α-radioligand therapy of patients with extensive skeletal metastases of castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2022 Aug;49(10):3581-3592. doi: 10.1007/s00259-022-05778-w. Epub 2022 Apr 6. PMID: 35384462.
Metebi A, Kauffman N, Xu L, Singh SK, Nayback C, Fan J, Johnson N, Diemer J, Grimm T, Zamiara M, Zinn KR. Pb-214/Bi-214-TCMC-Trastuzumab inhibited growth of ovarian cancer in preclinical mouse models. Front Chem. 2024 Jan 25;11:1322773. doi: 10.3389/fchem.2023.1322773. PMID: 38333550; PMCID: PMC10850308.
Video “Alpha is More Than Just Ac-225: Pb-212” JNM Podcast. Ken Herrmann, MD; David Bauer, PhD, from Memorial Sloan Kettering Cancer Center; Jane Sosabowski, BSc, MSc, PhD, from Queen Mary, University of London; Nick Fletcher, PhD, from the University of Queensland, Australia.
Demirci E, Alan Selçuk N, Beydağı G, Ocak M, Toklu T, Akçay K, Kabasakal L. Initial Findings on the Use of [225Ac]Ac-DOTATATE Therapy as a Theranostic Application in Patients with Neuroendocrine Tumors. Mol Imaging Radionucl Ther. 2023 Oct 20;32(3):226-232. doi: 10.4274/mirt.galenos.2023.38258. PMID: 37870290; PMCID: PMC10600558.
Stallons TAR, Saidi A, Tworowska I, Delpassand ES, Torgue JJ. Preclinical Investigation of 212Pb-DOTAMTATE for Peptide Receptor Radionuclide Therapy in a Neuroendocrine Tumor Model. Mol Cancer Ther. 2019 May;18(5):1012-1021. doi: 10.1158/1535-7163.MCT-18-1103. Epub 2019 Mar 29. PMID: 30926632; PMCID: PMC6497546.
Sabrina Serani, February 12, 2024, FDA Grants Breakthrough Therapy Designation to Novel Agent in GEP-NETs, retrieved 03.06.2024 from https://www.targetedonc.com/view/fda-grants-breakthrough-therapy-designation-to-novel-agent-in-gep-nets