TheraP study comparing radioligand treatment with 177Lu-PSMA-617 to the chemotherapy using cabazitaxel
Trial comparing radioligand treatment with 177Lu-PSMA-617 to the chemotherapy using cabazitaxel in treating mCRPC
TheraP, a phase II trial comparing radioligand treatment with 177Lu-PSMA-617 to the chemotherapy using cabazitaxel was finalized with 177Lu-PSMA demonstrating a clear advantage over the chemotherapy.
Probands’ selection criteria
200 men with mCRPC, overwhelmingly ADT-pretreated (91%), were selected based on 68Ga-PSMA PET/CT and 18F-FDG PET/CT. 68Ga-PSMA PET/CT was meant to detect the sufficiency of PSMA (min Standardized Uptake Value >20) in the tumor and metastases, a prerequisite for the radioligand therapy; otherwise, the radioligands have nowhere to dock to put it plainly. 18F-FDG PET/CT shows the glucose metabolism of the tumor and its metastases: high metabolism signals the aggressivity of cancer. Patients with lesions with discordant 68Ga-PSMA PET/CT and 18F-FDG PET/CT pictures, meaning aggressive lesions with insufficient PSMA, were also excluded. After random assignment, 98 men were assigned to 177Lu-PSMA-617 (6·0–8·5 GBq intravenously every 6 weeks for up to six cycles), and 85 – to cabazitaxel (20 mg/m² intravenously every 3 weeks for up to ten cycles).
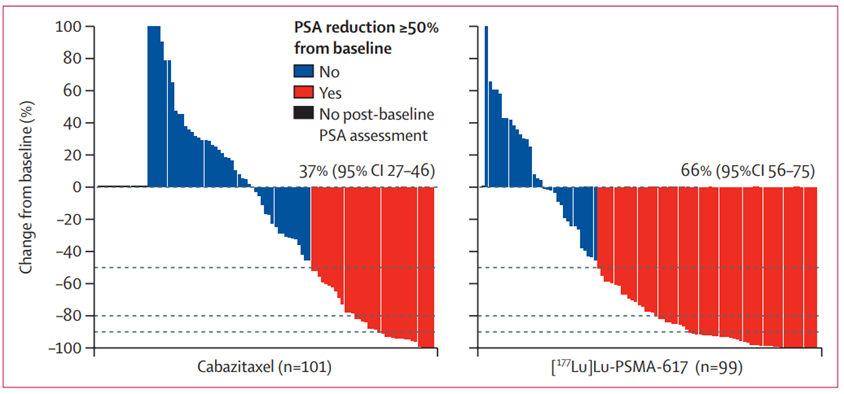
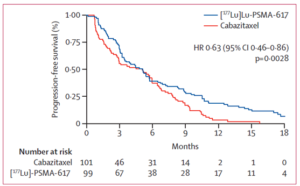
Findings
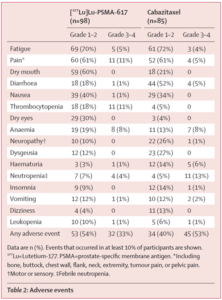 |
Grade 3–4 adverse events occurred in 32 (33%) of 98 men in the 177Lu-PSMA-617 group versus 45 (53%) of 85 men in the cabazitaxel group. |
Discussion
This study provides complementary data to the VISION trial because cabazitaxel was not included in the standard-of-care group in the VISION trial. Both trials used ⁶⁸Ga-PSMA-11 for patient selection, with TheraP using an additional quantitative PET parameter (uptake SUVmax) and ¹⁸F-FDG to identify patients with discordant ¹⁸F-FDG-positive, PSMA-negative lesions.
It should be noted that prostate cancer amongst the probands was really advanced – 85% had over 20 metastatic lesions. The efficacy and safety profile of 177Lu-PSMA-617 demonstrated in all trials so far has generated interest in exploring the use of 177Lu-PSMA earlier in the course of the disease. Multiple trials are underway including 177Lu-PSMA combined with immune checkpoint inhibitors, a poly(ADPribose) polymerase inhibitor, or enzalutamide. Use of 177Lu-PSMA up-front in men with newly diagnosed metastatic hormone-sensitive prostate cancer is also being explored.
The ability to select patients who are most likely to benefit from 177Lu-PSMA therapy is a key advantage of the theranostic approach of combining imaging and therapy modalities.
Bibliography (source of all tables)
Hofman M., Louise Emmett L., Sandhu S., Iravani A., Joshua A., Goh J., et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. The Lancet 397, issue 10276, P797-804, February 27, 2021 https://doi.org/10.1016/S0140-6736(21)00237-3