FAPI
Nuclear theranostics: what’s next?
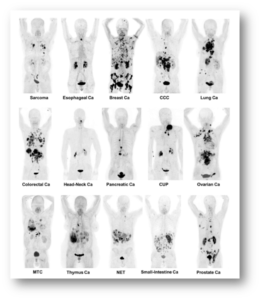
While theranostics has immensely improved overall survival and quality of life of NET and prostate cancer patients and is increasingly becoming mainstream, the science is tirelessly working on the next big thing – pan-cancer theranostics based on fibroblast activation protein (FAP) found in the cancer-associated fibroblasts (CAF). With many widespread cancers in question, this would be truly revolutionary. First-in-human PET/CT studies of 68Ga-FAPI have demonstrated excellent results. A tracer derivative better suited for the therapeutical ends is within reach.
What are cancer-associated fibroblasts (CAFs)?
Tumor masses consist of cancer cells but also vascular structures, inflammatory cells, fibroblasts (the most common type of cell in connective tissue), and collagen that together make up the tumor stroma. Several highly prevalent cancers, such as breast, colon, and pancreatic carcinomas, are characterized by a strong growth of fibrous tissue in particular around the tumor. The stroma can account for up to 90% of the mass in such cancers. It has been detected that these cancer-associated fibroblasts (CAFs) express a so-called fibroblast activation protein (FAP). Its expression in normal adult tissues is absent or low.
CAFs’ role in cancer proliferation
CAFs, especially the subtypes expressing the FAP, have been reported not only to physically support cancer cells but also promote cancer growth, invasion and resistance to the body’s own immune response as well as to radiation and chemotherapy. They are the key players in tumor ability to form new blood cells (angiogenesis) and ensure its supply with nutrients. CAFs produce several growth factors that lead to tumor formation, proliferation and metastasis (such as vascular endothelial growth factor (VEGF) or fibroblast growth factor (FGF)).[i]
| Hence, FAP presents an ideal pan-cancer target structure for imaging and targeted delivery of therapeutics. It opens up new applications for non-invasive tumor visualization, staging examinations, and radioligand therapy for many cancers that have not yet been covered by nuclear theranostics and can be poorly treated by other therapies. In fact, besides thyroid cancer currently prostate cancer and neuroendocrine tumors are its predominant beneficiaries. “Targeting FAP to deplete stromal CAFs may disrupt cancer supportive functions and inhibit cancer growth. Furthermore, by breaking the stroma barrier, the effectiveness of other pharmacologic, immunologic, radiation- or cell-based systemic therapies may thus be enhanced.”[ii]
So much about the lock. The challenge lies in developing a suitable key. |
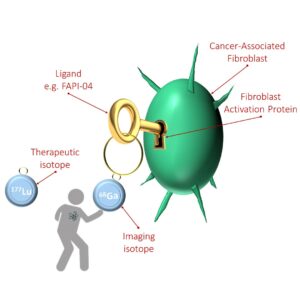 |
Tracers
FAP-specific inhibitors were first developed in the early 2000s as anticancer drugs, such as sibrotuzumab or talabostat. Inhibitor-based radiopharmaceuticals are the next step. Scientists are intensively working on the development of a theranostic tracer that can be employed for both diagnostics and therapy; that is, be labelled with an imaging or therapeutic isotope (e.g. gallium-68 68Ga or lutetium-177 177Lu respectively), dock to FAP upon being injected and stay there long enough to produce the desired effect.
Imaging
Radioligand therapy
Whereas the tumor uptake of the radiopharmaceutical is more important for diagnostic means, retention time of the tracer in the fibroblast becomes crucial for the therapeutic ends. The radiation emitter must stay in the cell long enough for it to cause an effect, i.e. destroy the cell. FAPI-04 washes out of the tumor tissue relatively rapidly and limits the delivery of the radiation dose of the common therapeutic emitters, like lutetium or actinium. Alternatively, “the physical half-life of the therapeutic isotope needs to be matched to the tumor retention time.”[ii]
Limitations
Despite FAPI excellent tumor-to-background contrast ratios ranging from blood to liver to muscles to intestines, FAP expression is not exclusively cancer-specific. It still shows in some non-cancerous tissues, whenever fibroblasts become activated, for example “in remodelling processes such as wound healing, inflammation, or fibrosis”[ii].
Conclusion
Many cancers “tend to grow in an invasive and diffuse manner infiltrating the adjacent often delicate and anatomically complex structures”[i] FAPI-based imaging and radiotherapy will constitute a quantum leap in pan-cancer nuclear theranostics. It will enable higher precision in diagnostics and treatment of many cancer types. Scientists continue tinkering on the tracer to optimize the uptake and retention. Tests with a variant FAPI-46 have been encouraging. Another novel compound has also showed promising results in patients with iodine refractory thyroid cancer and demonstrated significantly longer retention of over several days in the tumor and metastases.[iii] These are the latest, but for sure not the last tracer derivatives. Stay tuned.
Endnotes
[i] Syed, M., Flechsig, P., Liermann, J. et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur J Nucl Med Mol Imaging 47, 2836–2845 (2020). https://doi.org/10.1007/s00259-020-04859-y
[ii] Calais, Jérémie. (2020). FAP: The Next Billion Dollar Nuclear Theranostics Target?. Journal of Nuclear Medicine. 61. jnumed.119.241232. 10.2967/jnumed.119.241232.
[iii] Ballal S, Yadav MP, Moon ES, Roesch F, Kumari S, Agarwal S, Tripathi M, Sahoo RK, Mangu BS, Tupalli A, Bal C. Novel Fibroblast Activation Protein Inhibitor-Based Targeted Theranostics for Radioiodine-Refractory Differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid. 2022 Jan;32(1):65-77. doi: 10.1089/thy.2021.0412. Epub 2021 Dec 31. PMID: 34641705.
Figure 1 J Nucl Med 2019; 60:801–805, DOI: 10.2967/jnumed.119.227967
Bibliography
Syed, M., Flechsig, P., Liermann, J. et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur J Nucl Med Mol Imaging 47, 2836–2845 (2020). https://doi.org/10.1007/s00259-020-04859-y
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, Adeberg S, Rathke H, Röhrich M, Winter H, Plinkert PK, Marme F, Lang M, Kauczor HU, Jäger D, Debus J, Haberkorn U, Giesel FL. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med. 2019 Jun;60(6):801-805. doi: 10.2967/jnumed.119.227967. Epub 2019 Apr 6. PMID: 30954939; PMCID: PMC6581228
Giesel FL, Heussel CP, Lindner T, Röhrich M, Rathke H, Kauczor HU, Debus J, Haberkorn U, Kratochwil C. FAPI-PET/CT improves staging in a lung cancer patient with cerebral metastasis. Eur J Nucl Med Mol Imaging. 2019 Jul;46(8):1754-1755. doi: 10.1007/s00259-019-04346-z. Epub 2019 May 22. PMID: 31119317
Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, Kratochwil C, Debus J, Marmé F, Jäger D, Mier W, Haberkorn U. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J Nucl Med. 2019 Oct;60(10):1421-1429. doi: 10.2967/jnumed.118.224469. Epub 2019 Mar 8. PMID: 30850501; PMCID: PMC6785792
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, Jäger D, Mier W, Haberkorn U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J Nucl Med. 2018 Sep;59(9):1415-1422. doi: 10.2967/jnumed.118.210443. Epub 2018 Apr 6. PMID: 29626119
Calais, Jérémie. (2020). FAP: The Next Billion Dollar Nuclear Theranostics Target?. Journal of Nuclear Medicine. 61. jnumed.119.241232. 10.2967/jnumed.119.241232
Ballal S, Yadav MP, Moon ES, Roesch F, Kumari S, Agarwal S, Tripathi M, Sahoo RK, Mangu BS, Tupalli A, Bal C. Novel Fibroblast Activation Protein Inhibitor-Based Targeted Theranostics for Radioiodine-Refractory Differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid. 2022 Jan;32(1):65-77. doi: 10.1089/thy.2021.0412. Epub 2021 Dec 31. PMID: 34641705