Double Punch Against Hidden Prostate Cancer: Lutetium-177 + Terbium-161 vs Undetectable Micrometastases
Double Punch Against Hidden Prostate Cancer: Lutetium-177 + Terbium-161 vs Undetectable Micrometastases
Recent advances in PSMA-targeted radioligand therapy (RLT) highlight its expanding role in eradicating occult micrometastatic disease in prostate cancer.
Lutetium-177: Winning Early Battles Against Limited Recurrences
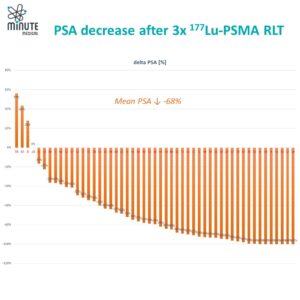
The phase II LUNAR trial in a few-site recurrent hormone-sensitive prostate cancer demonstrated that adding two cycles of 177Lu-PNT2002 before high-dose external radiation (stereotactic ablative body radiotherapy or SABR) to the visible metastases significantly extended progression-free survival versus SABR alone (from about 7 months to over 17 months). It was very well tolerated, with hardly any extra side effects. Such success is attributed to the comprehensive targeting of PSMA-expressing micrometastases undetected by conventional imaging.
Terbium-161: The Next-Gen Supercharged Radionuclide
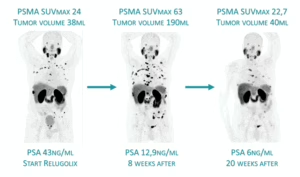
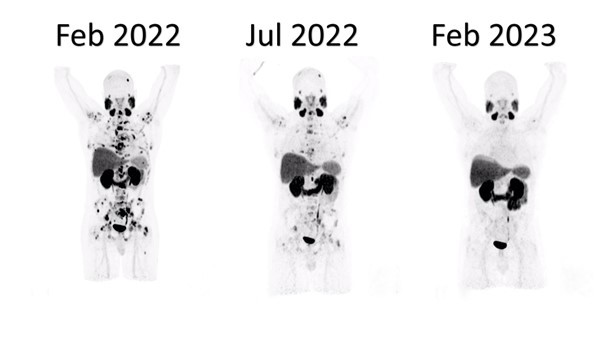
Building on this foundation, emerging data with 161Tb-PSMA radioligands—led by ongoing phase I/II (VIOLET) and pilot/registry studies at Saarland University—reveal superior radiation action profile of Terbium-161 over Lutetium-177. 161Tb emits comparable medium-energy beta particles but adds abundant high-linear-energy-transfer conversion and Auger electrons, depositing markedly higher absorbed radiation doses in single cells and micrometastatic clusters (up to several-fold greater nuclear doses in preclinical models). This enhanced cytotoxicity is particularly potent against small-volume, undetectable disease, yielding promising response and tumor control even in heavily pretreated mCRPC patients progressing after standard 177Lu-PSMA RLT.
The 1-2 Knockout: Lu-177 + Tb-161
Collectively, these developments position PSMA RLT as a cornerstone for micrometastasis-directed therapy across disease stages, with Terbium-161 representing a next-generation upgrade. A smart early-stage combination plan —such 1–2 cycles of ¹⁷⁷Lu-PSMA followed by ¹⁶¹Tb-PSMA—could use Lutetium-177 to target the larger tumor sites, while leveraging ¹⁶¹Terbium’s augmented potency to eliminate resistant micrometastatic reservoirs more thoroughly and potentially achieve longer-lasting results.
At our clinic, we apply this precise combination protocol to give patients the best chance of targeting both visible and hidden cancer sites.
Bibliography
Buteau JP, Kostos L, Jackson PA, Xie J, Haskali MB, Alipour R, McIntosh LE, Emmerson B, MacFarlane L, Martin CA, Chan J, Williams SE, Jewell KE, Eifer M, Hamilton AJ, Harris WQ, Akhurst T, Au L, Cardin AJ, Furic L, Kashyap RK, Kong G, Ravi Kumar AS, Murphy DG, Ravi R, Saghebi J, Sandhu S, Tran B, Azad AA, Hofman MS. First-in-human results of terbium-161 [161Tb]Tb-PSMA-I&T dual beta-Auger radioligand therapy in patients with metastatic castration-resistant prostate cancer (VIOLET): a single-centre, single-arm, phase 1/2 study. Lancet Oncol. 2025 Aug;26(8):1009-1017. doi: 10.1016/S1470-2045(25)00332-8. Epub 2025 Jul 3. PMID: 40617237.
Amar U. Kishan et al.177Lu-Prostate-Specific Membrane Antigen Neoadjuvant to Stereotactic Ablative Radiotherapy for Oligorecurrent Prostate Cancer (LUNAR): An Open-Label, Randomized, Controlled, Phase II Study. J Clin Oncol 0, JCO-25-01553 DOI:10.1200/JCO-25-01553
Rosar, F., Burgard, C., Petrescu, C., Blickle, A., Bartholomä, M., Maus, S., Bastian, M.B., Speicher, T., Schaefer-Schuler, A., Ezziddin, S. (2025). Pilot experience of [161Tb]Tb-PSMA-617 RLT in mCRPC patients after conventional PSMA RLT within a prospective registry. Theranostics, 15(17), 9019-9028. https://doi.org/10.7150/thno.115831.
“Augerlicious Terbium 161 Double Punch to Cancer Cells - Global Knowledge Exchange Webinar” ProsTIC TV
Schaefer-Schuler A, Burgard C, Blickle A, Maus S, Petrescu C, Petto S, Bartholomä M, Stemler T, Ezziddin S, Rosar F. [161Tb]Tb-PSMA-617 radioligand therapy in patients with mCRPC: preliminary dosimetry results and intra-individual head-to-head comparison to [177Lu]Lu-PSMA-617. Theranostics. 2024 Feb 24;14(5):1829-1840. doi: 10.7150/thno.92273. PMID: 38505615; PMCID: PMC10945337.
Rosar F, Maus S, Schaefer-Schuler A, Burgard C, Khreish F, Ezziddin S. New Horizons in Radioligand Therapy: 161Tb-PSMA-617 in Advanced mCRPC. Clin Nucl Med. 2023 May 1;48(5):433-434. doi: 10.1097/RLU.0000000000004589. Epub 2023 Feb 8. PMID: 36758550.
Tschan VJ, Busslinger SD, Bernhardt P, Grundler PV, Zeevaart JR, Köster U, van der Meulen NP, Schibli R, Müller C. Albumin-Binding and Conventional PSMA Ligands in Combination with 161Tb: Biodistribution, Dosimetry, and Preclinical Therapy. J Nucl Med. 2023 Oct;64(10):1625-1631. doi: 10.2967/jnumed.123.265524. Epub 2023 Jul 13. PMID: 37442604.