Upregulating PSMA: How Antihormonal Therapy Assists Radioligand Therapy
Upregulating PSMA: How Antihormonal Therapy Assists Radioligand Therapy
PSMA-directed radioligand therapy (RLT) — using isotopes such as Lutetium-177, Actinium-225, or Terbium-161 — has shown impressive effectiveness in treating prostate cancer, with a favourable safety profile. Its success has led to earlier integration into treatment protocols.
Yet, a certain proportion of prostate cancer cells, particularly in heavily pretreated patients, show insufficient PSMA expression, making them ineligible for RLT based on current imaging and biomarker thresholds.
The good news is that low PSMA expression isn’t necessarily a dead end. It can be pharmacologically upregulated — opening the door for these patients to benefit from RLT after all. Let’s dive into it.
What regulates PSMA?
In normal (benign) prostate tissue, PSMA expression is low. Despite its misleading name (Prostate-Specific Membrane Antigen), it is neither truly prostate-specific nor particularly abundant in prostate cells. The name arose simply because it was first discovered on the surface of prostate cells; the accurate designation is Folatehydrolase.
Androgen receptor (AR) signalling — meaning when male hormones (androgens) like testosterone or dihydrotestosterone (DHT) bind to androgen receptors on cell surfaces, particularly in the prostate — suppresses PSMA expression by downregulating the FOLH1 gene.
What works in one direction also works in the other: when testosterone is absent (due to drug suppression or receptor blockade), the FOLH1 gene — including its androgen-binding site — is upregulated, leading to a (temporary) overexpression of PSMA. This effect is most pronounced in cells with initially low PSMA expression, as shown in preclinical experiments (Impact of Androgen Receptor Activity on Prostate-Specific Membrane Antigen Expression in Prostate Cancer Cells - PubMed).
Prostate cancer, AR signalling, and PSMA: an intricate interplay
Hormone-sensitive prostate cancer depends on AR signalling for tumor survival and proliferation. That’s why one of the first-line treatments is to shut this pathway down — either by:
- Lowering testosterone levels (e.g., with GnRH antagonists like Orgovyx), or
- Blocking the AR directly (e.g., with AR inhibitors like enzalutamide or apalutamide).
In these early stages of hormone-sensitive cancer, PSMA expression can temporarily increase when androgen deprivation therapy (ADT) is initiated (see mechanism above). However, after several weeks of treatment, PSMA levels tend to decline again, because most cells undergo apoptosis (programmed cell death) in the absence of testosterone.
Unfortunately, a subset of cancer cells survives this testosterone deprivation and eventually become castration-resistant as an evolutionary response to ADT. At this stage, tumors often become more PSMA-avid again, making them once more suitable targets for radioligand therapy. This occurs because the PSMA gene FOLH1 remains overexpressed without AR signalling. Thus, PSMA upregulation is essentially a byproduct of the adaptive mechanisms that allow cancer cells to survive under treatment pressure. The resistant cells that overcome androgen deprivation multiply and thrive, which ultimately renders ADT ineffective.
In these cases, initiating androgen receptor pathway inhibitor (ARPI) therapy is the standard approach, although the benefit is limited, since the androgen receptor is no longer the primary driver of cancer growth. However, the resulting PSMA upregulation persists — and this creates a vulnerability we can exploit.
A new paradigm: combining AR-pathway suppression with RLT
That’s exactly what recent clinical studies are exploring: identifying and targeting, at an early stage, those adaptive cancer cells that survive ADT — potentially disrupting the tumor’s escape mechanisms. The results are promising: many patients show a notable rise in PSMA expression shortly after initiating AR blockade, potentially converting a "PSMA-low–expressing" patient into a candidate for RLT.
A recent proof of this concept in the initial hormone-sensitive stages was presented at ASCO 2025, with the first results of the PSMAddition trial, which demonstrated better outcomes for RLT plus ADT compared to ADT alone.
This synergy between hormonal blockade and PSMA targeting may mark a new chapter in personalized prostate cancer therapy and shift its use to very early stages of the disease.
Our clinical experience
At our clinic, we have been applying this concept in practice for many years. We successfully use initial androgen deprivation therapy in selected patients to stimulate PSMA expression ahead of planned radioligand therapy — with excellent results. This preparatory step can be a game-changer for patients who show heterogeneous or low PSMA expression on initial staging PSMA PET.
Summing up:
- Low PSMA expression is not irreversible.
- Androgen pathway suppression (through lowering testosterone or using AR inhibitors) can induce PSMA upregulation.
- This opens new opportunities for early RLT — even in patients who were initially deemed unsuitable.
- Combining AR-targeted therapies with PSMA-targeted therapies may provide additive and possibly synergistic clinical benefits.
As treatment strategies continue to evolve, the interplay between hormonal manipulation and molecular nuclear therapy is emerging as a powerful tool in the fight against prostate cancer.
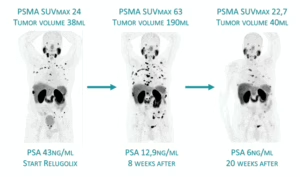
Sequential PSMA PET in a patient newly starting ADT, demonstrating striking PSMA overexpression after 8 weeks, despite a significant decrease in PSA levels (PSMA PET pseudoprogression in PSMA volume and true progression in PSMA uptake).






